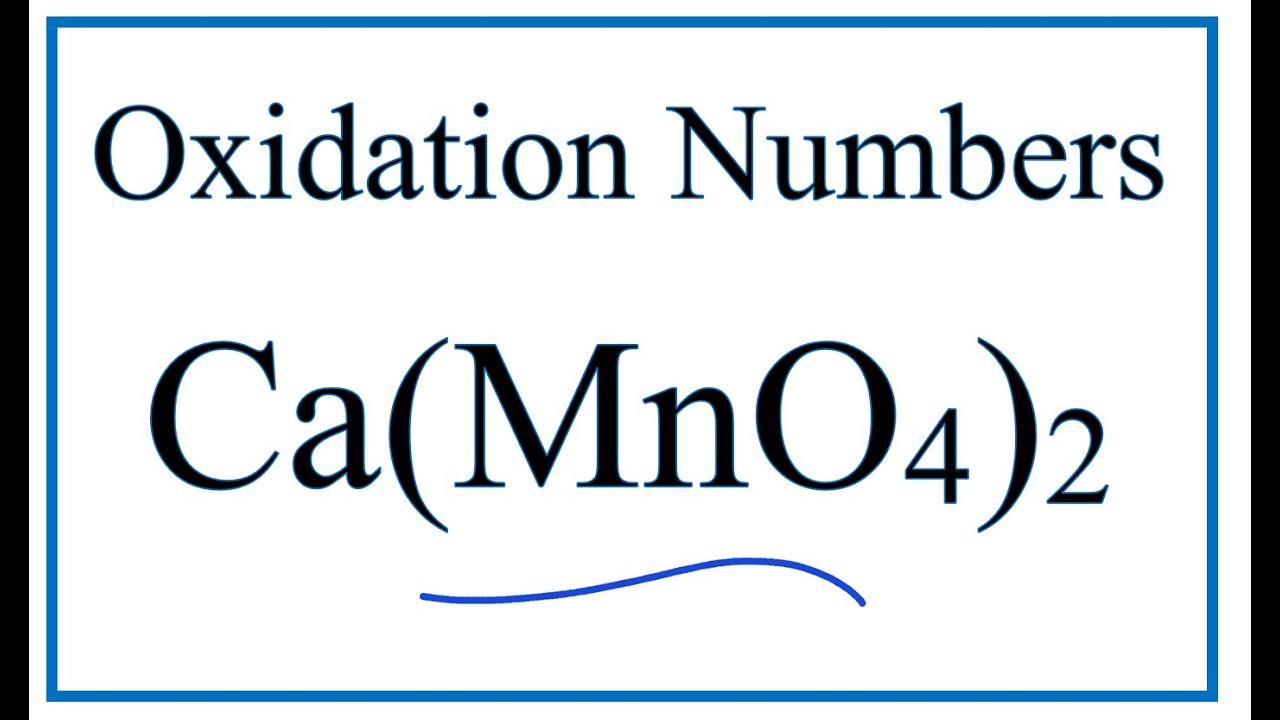
Iron(iii) permanganate fe(mno4)3 molar mass, molecular weight When it is bonded to fluorine (f) it has an oxidation number of +2. Here it is bonded to mn so the oxidation. Molar mass of fe (mno4)3 molar mass, molecular weight and elemental composition calculator molar mass of fe (mno4)3 is 412. 6519 g/mol get control of 2022!
Madre Tierra Carta, 5.26 MB, Carta de la Madre Tierra, 03:50, 5,071, Club de Lectura ODS México. Leer sostiene, 2020-05-22T03:55:47.000000Z, 19, Cuento de la madre tierra, es.slideshare.net, 728 x 1043, jpeg, tierra madre carta madretierra, 20, madre-tierra-carta, Zadania lekcyjne
Convert between fe (mno4)3 weight and moles elemental composition of fe (mno4)3 formula in hill system is femn3o12 Berechnen sie das gewicht von fe (mno4)3 oder mol elementare zusammensetzung von fe (mno4)3 formel hill ist femn3o12 berechnung der molaren masse (molares gewicht) um die molare masse einer chemischen verbindung zu berechnen, geben sie ihre formel ein und klicken sie auf berechnen. für die chemische formel können sie nutzen: To produce a balanced equation, we adds (i) and (ii) in such a way as to remove the electrons as virtual particles of convenience. 5 × (i) + (ii) gives. The mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: Grams = mole × molar mass. Molar mass of nacl is 58. 443, how many grams is 5 mole nacl? Grams = 58. 443 × 5 = 292. 215 (g) molar mass of agno3 is 169. 873, 2 kg agno3 is equal to how many moles? Moles = 2000 / 169. 873 = 11. 77 (moles)
Aquí How to find the Oxidation Number for Fe in Fe(NO3)3
Aquí Balancing Redox Reactions (Acidic Solution) Complex Example más
Videos Redox Titration between MnO4- and Fe2+ actualizar
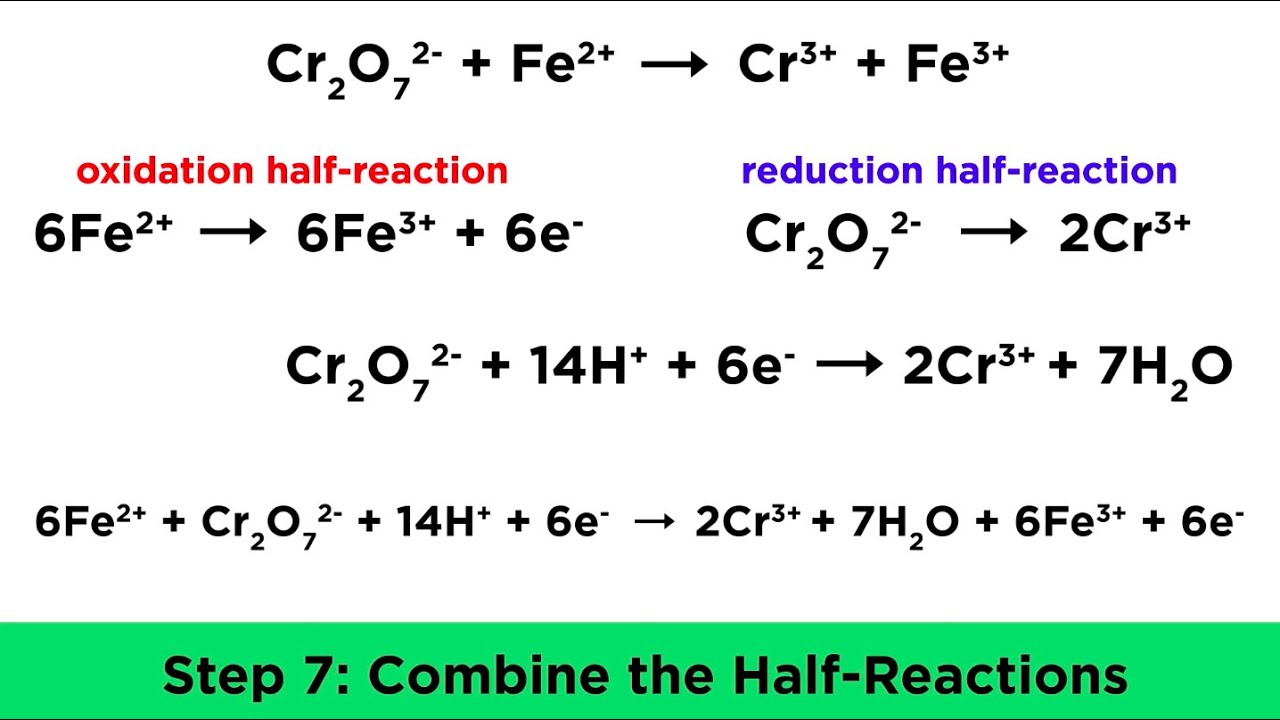
Acerca de Balancear por 𝐢𝐨𝐧 𝐞𝐥𝐞𝐜𝐭𝐫ó𝐧 𝐅𝐞(𝟐+) + 𝐌𝐧𝐎𝟒(−) → 𝐅𝐞(𝟑+) + 𝐌𝐧(𝟐+) en 𝐦𝐞𝐝𝐢𝐨 á𝐜𝐢𝐝𝐨 actualizar
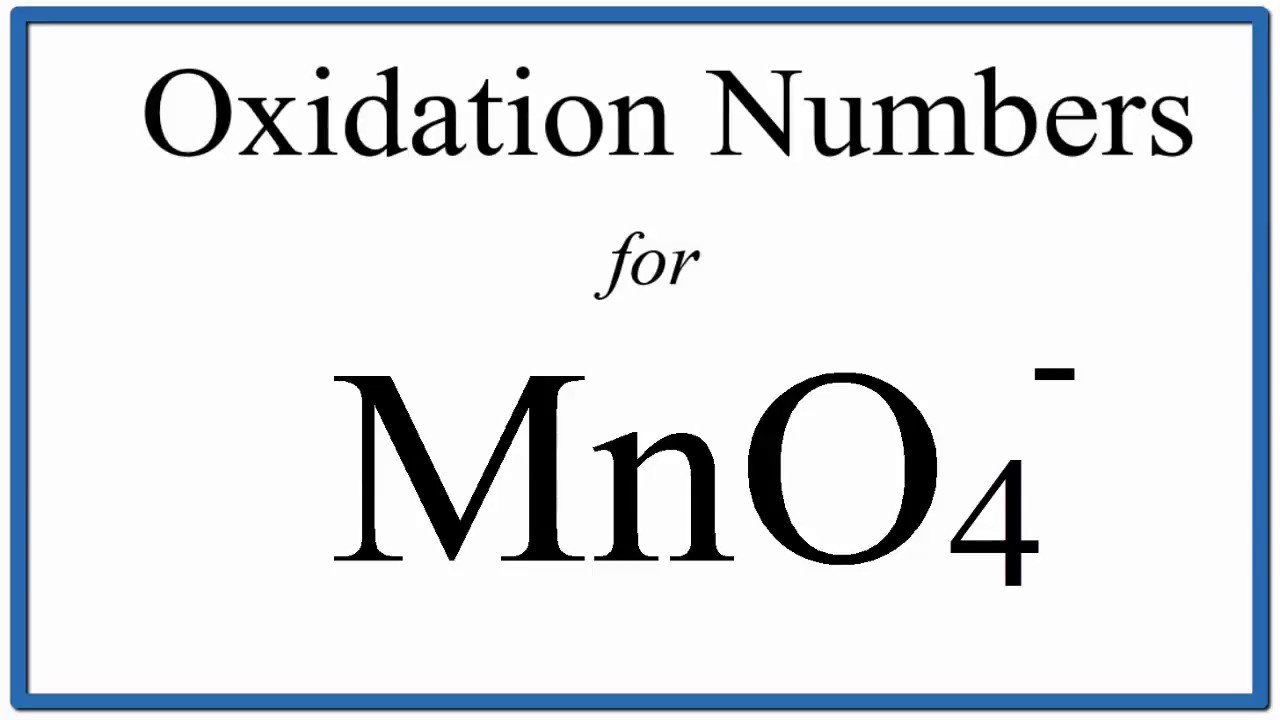
Viral How to find the Oxidation Number for Mn in the MnO4 – ion. actualizado
Viral balancing RedOx reactions Basic sol viral
Veamos Balancing Redox Reactions in Acidic and Basic Conditions actualizar
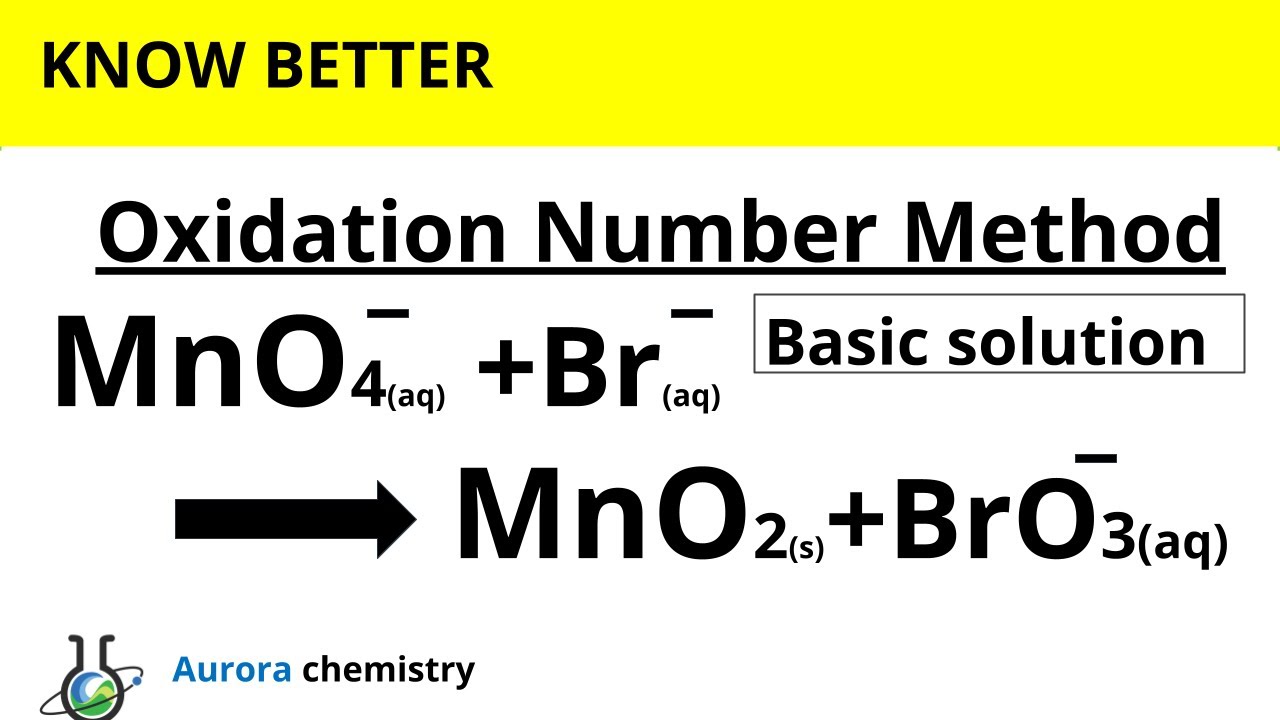
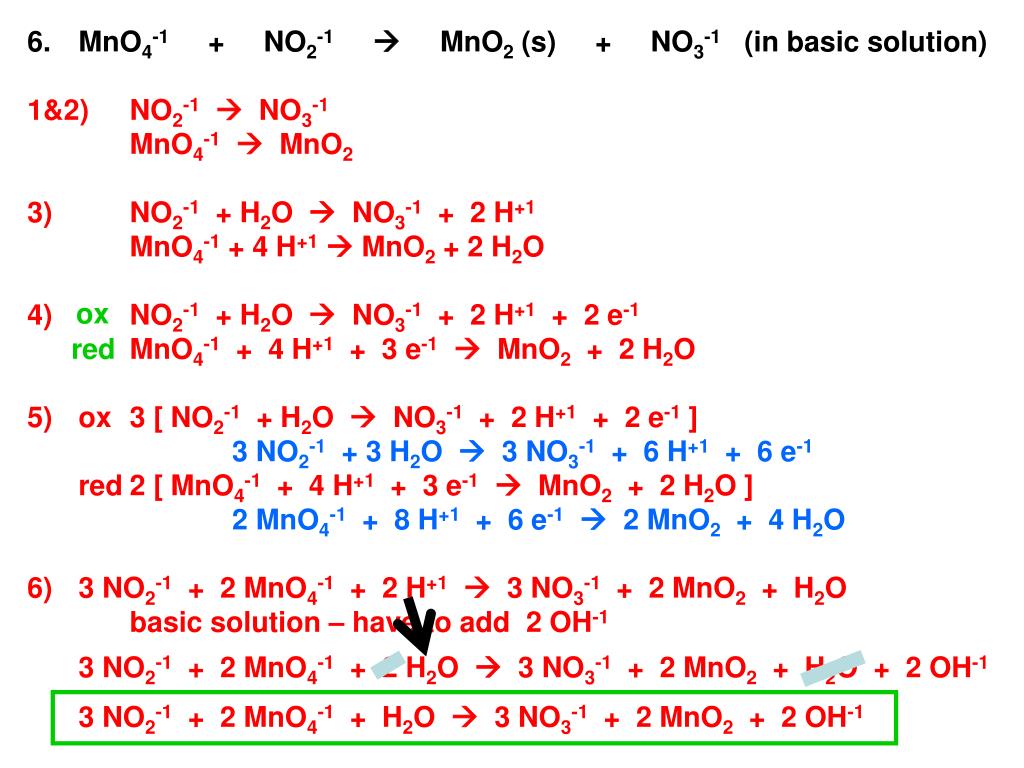
Acerca de Redox Balance MnO4- + Br- = MnO2 + BrO3- || Oxidation Number Method volviéndose viral
Temas How to find the Oxidation Number for Mn in Ca(MnO4)2 volviéndose viral
Redox Titration – Permanganate vs Iron(II) | Full Example + Calculations popular
Detalles de Fe Mno4 3 en su totalidad
To find the correct oxidation state of Fe in Fe(NO3)3 (Iron (III) nitrate), and each element in the molecule, we use a few rules and some simple math.
First, since the Fe(NO3)3 molecule doesn’t have an overall charge (like NO3- or H3O+) we could say that the total of the oxidation numbers for Fe(NO3)3 will be zero since it is a neutral molecule.
We write the oxidation number (O.N.) for elements that we know and use these to figure out oxidation number for Fe.
———-
GENERAL RULES
Free elements have an oxidation state of zero (e.g. Na, Fe, H2, O2, S8).
In an ion the all Oxidation numbers must add up to the charge on the ion.
In a neutral compound all Oxidation Numbers must add up to zero.
Group 1 = +1
Group 2 = +2
Hydrogen with Non-Metals = +1
Hydrogen with Metals (or Boron) = -1
Fluorine = -1
Oxygen = -2 (except in H2O2 or with Fluorine)
Group 17(7A) = -1 except with Oxygen and other halogens lower in the group
———-
We know that Oxygen usually is -2 with a few exceptions. When Oxygen is in a peroxide, like H2O2 (Hydrogen peroxide), it has a charge of -1. When it is bonded to Fluorine (F) it has an oxidation number of +1.
Here it is bonded to Fe so the oxidation number on Oxygen is -2. Using this information we can figure out the oxidation number for the element Fe in Fe(NO3)3.
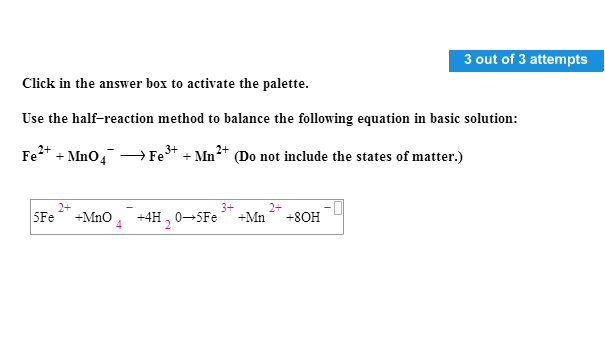
Acerca de Solved: Use The Half?reaction Method To Balance The Follow… | Chegg.com popular

Artículos PPT – Balancing Redox Equations PowerPoint Presentation, free download Nuevo
Nuevo PPT – Balancing Redox Equations PowerPoint Presentation – ID:2842629 más
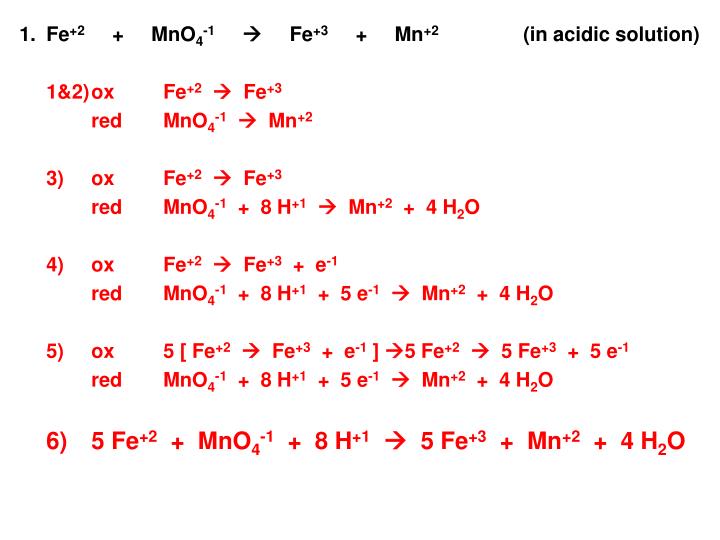
Aquí MnO4^- +Fe^2+ Mn^2+ + Fe^3+ Setarakan persamaan reaksi redoks berikut tendencias

Imprescindible Solved: 3) Balance The Following Redox Reaction If It Occu… | Chegg.com popular
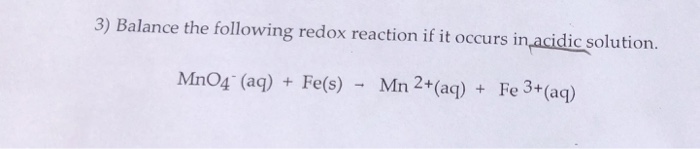
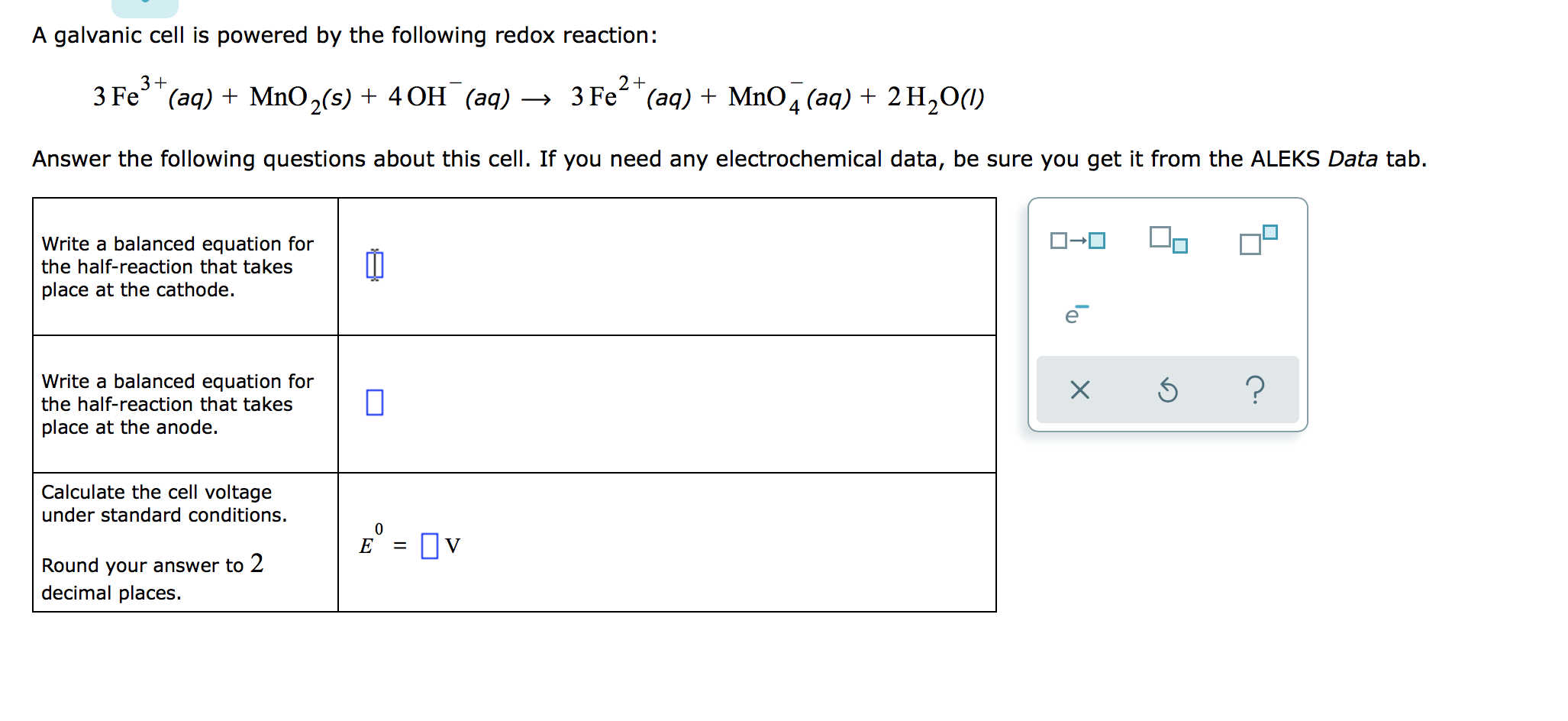
Aquí Solved: A Galvanic Cell Is Powered By The Following Redox | Chegg.com tendencias
Discusión Redox= quiz part 1 with answers Último
Reseñas PPT – REDOX REACTIONS PowerPoint Presentation, free download – ID:6527546 tendencias
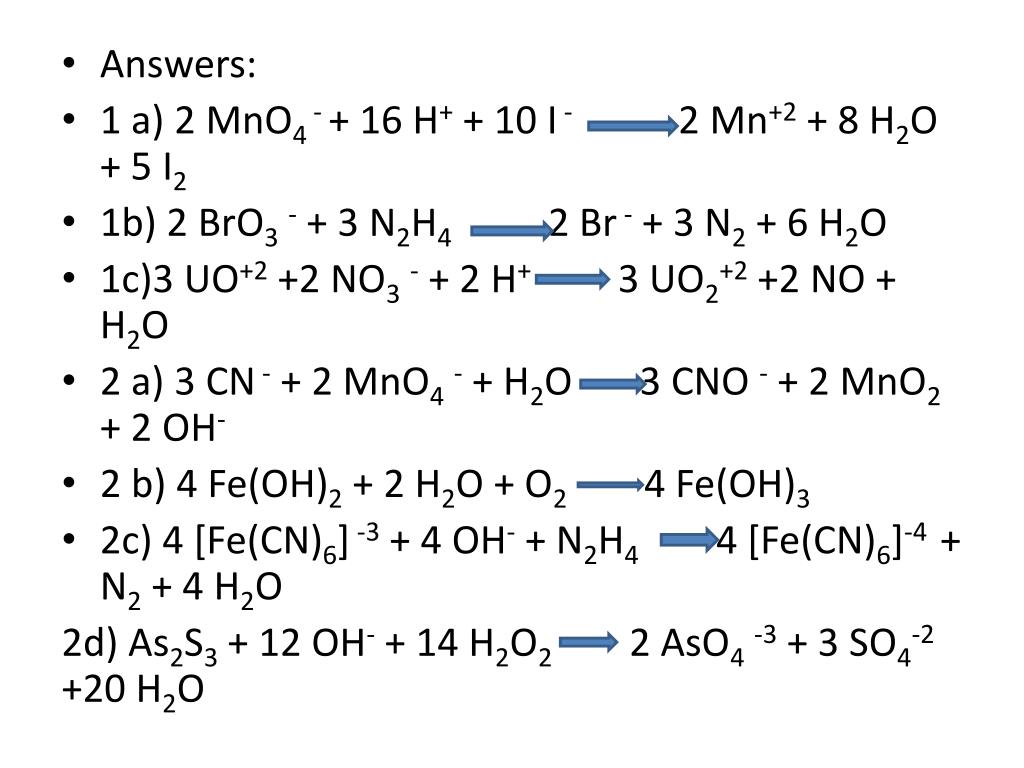
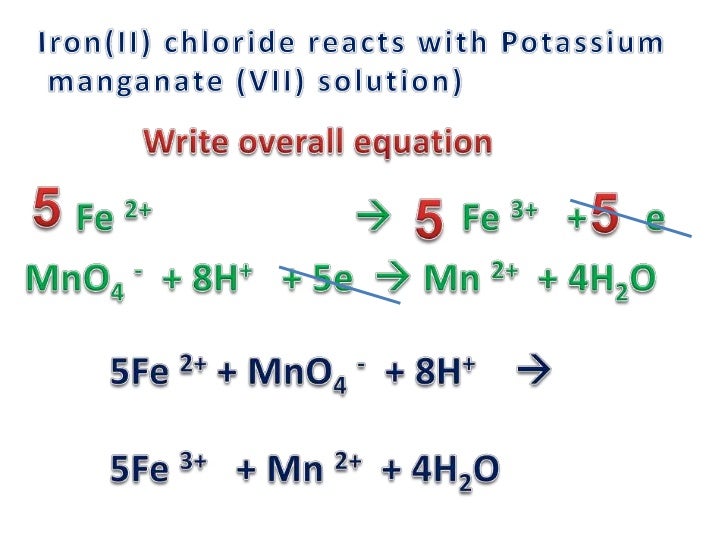
Noticias balance explain clearly oxidation state of fe3o4 fe3o4 mno4 h2o fe2o3 Nuevo
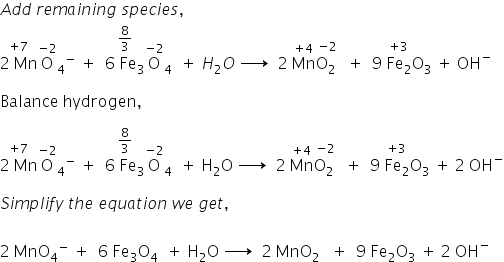
A
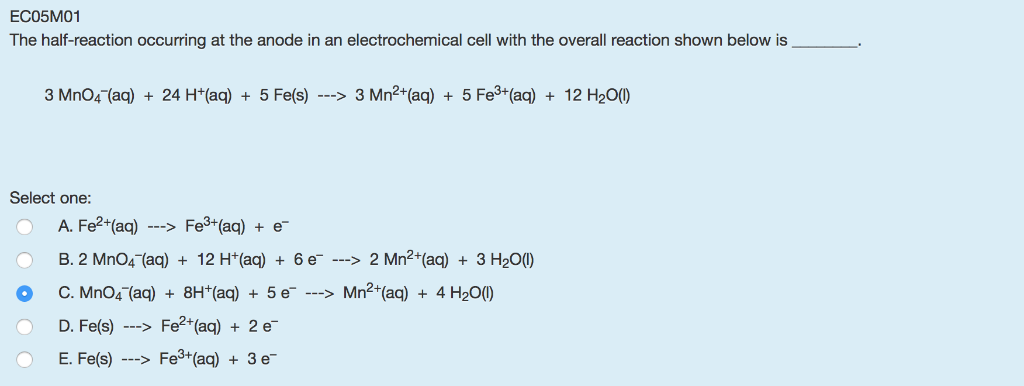
cerca de Solved: ECO5MO1 The Half-reaction Occurring At The Anode I… | Chegg.com más