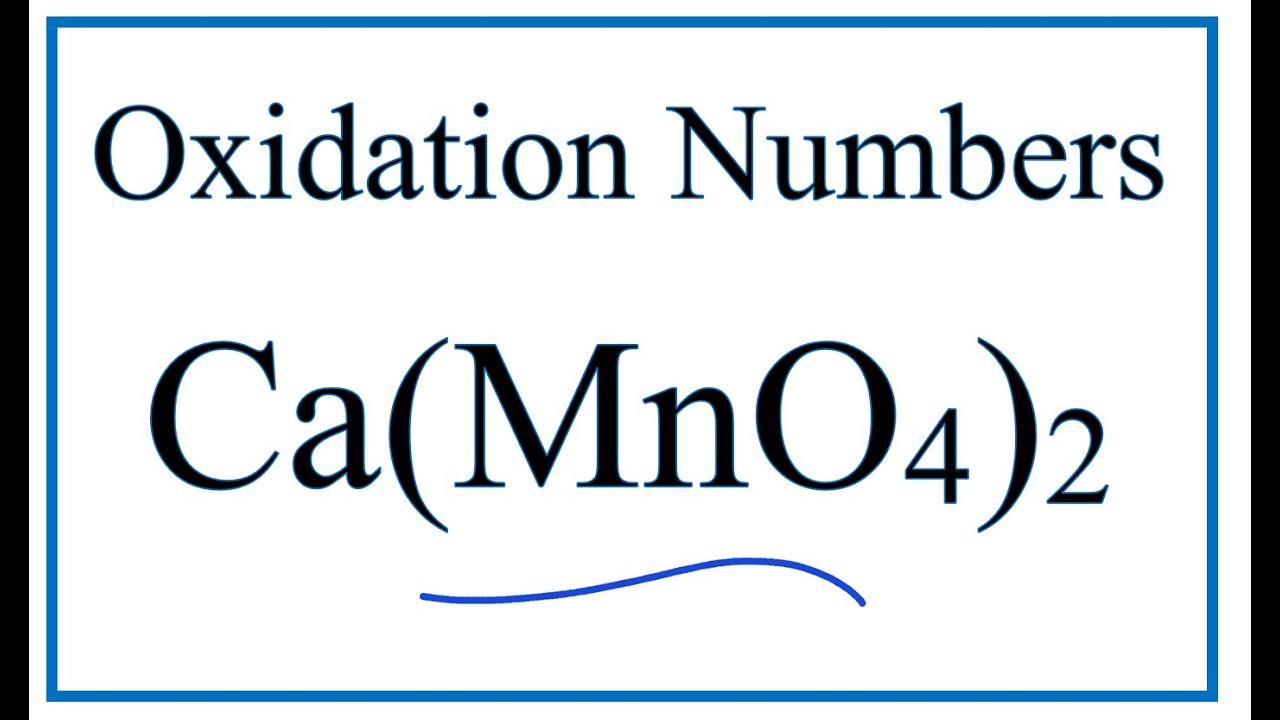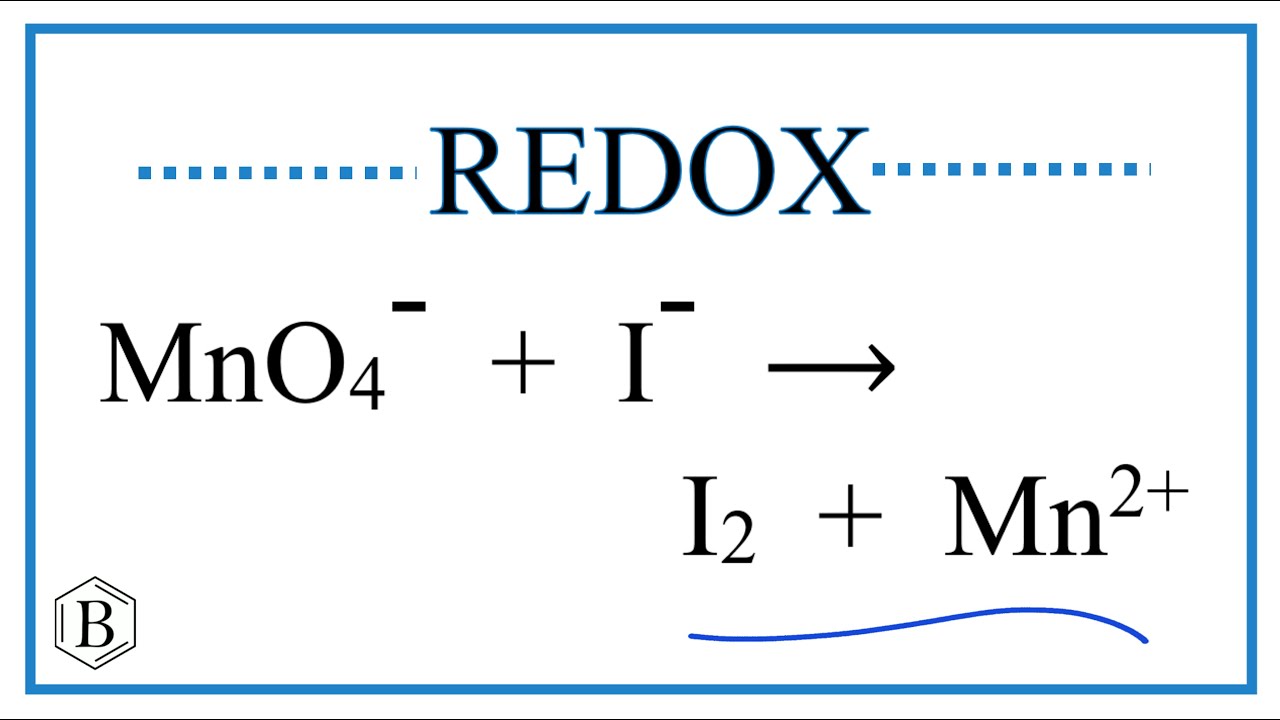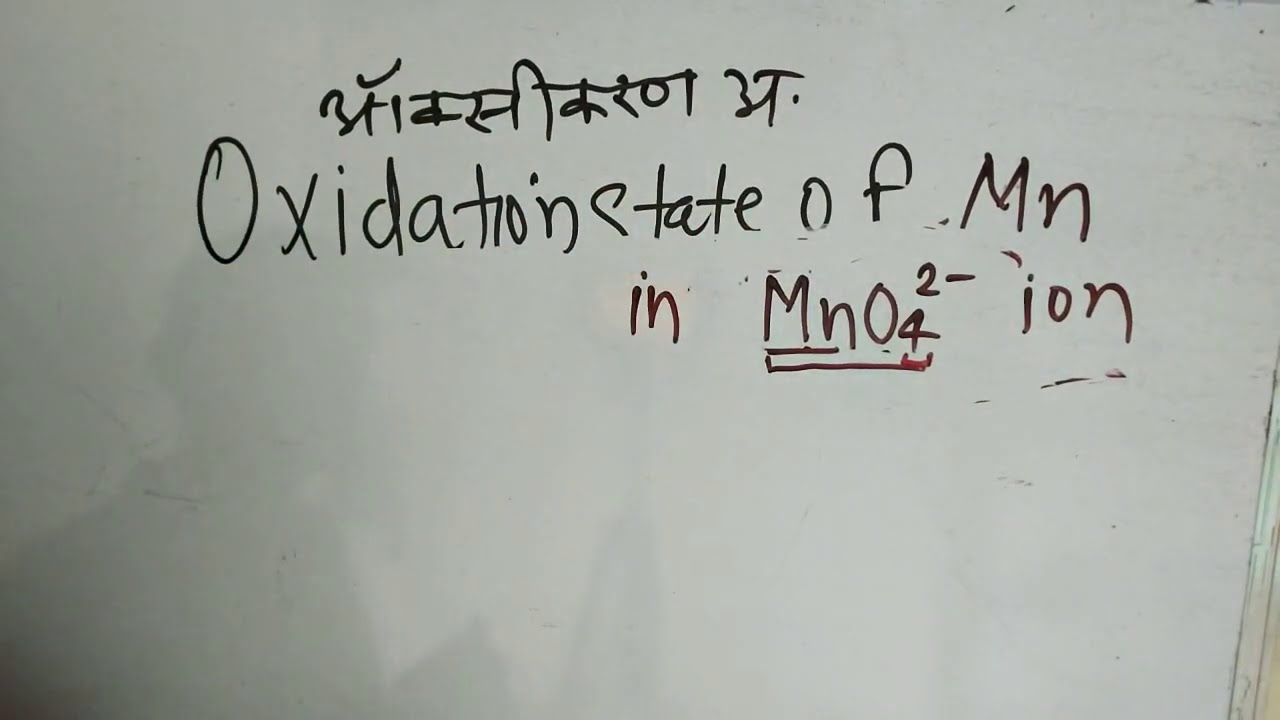
When it is bonded to fluorine (f) it has an oxidation number of +2. Here it is bonded to mn so the. Beryllium permanganate be(mno4)2 molar mass, molecular weight Barium permanganate ba(mno4)2 molar mass, molecular weight
Contar Vocales C++, 8.22 MB, 49. Programación en C++ || Cadenas || Ejercicio – Contando cada vocal en una frase, 05:59, 195,234, Programación ATS, 2016-05-13T01:52:30.000000Z, 19, Contar Vocales, Consonantes o Ambas en C, pdfslide.net, 1200 x 630, jpeg, , 20, contar-vocales-c, Zadania lekcyjne
Molar mass calculator also displays. Be(mno4)2 might be an improperly capitalized: Be(mno4)2 instructies en voorbeelden hieronder kan helpen om dit probleem op te lossen u kunt altijd om hulp vragen in het forum. Molar mass of be (mno4)2 molar mass, molecular weight and elemental composition calculator molar mass of be (mno4)2 is 246. 8835 g/mol get control of 2022! Track your food intake,. Ba (mno4)2 molecular weight molar mass of ba (mno4)2 = 375. 198298 g/mol this compound is also known as barium permanganate. Convert grams ba (mno4)2 to moles or moles ba. Dit is de eerste vergelijking die wordt gegeven:
Actualmente – How to find the Oxidation Number for Mn in Ca(MnO4)2
Acerca de How to find the Oxidation Number for Mn in Mg(MnO4)2 (Magnesium permanganate ) tendencias
Noticias 4.41b | Balance: MnO42−(aq) → MnO4−(aq) + MnO2(s) (in base) actualizar
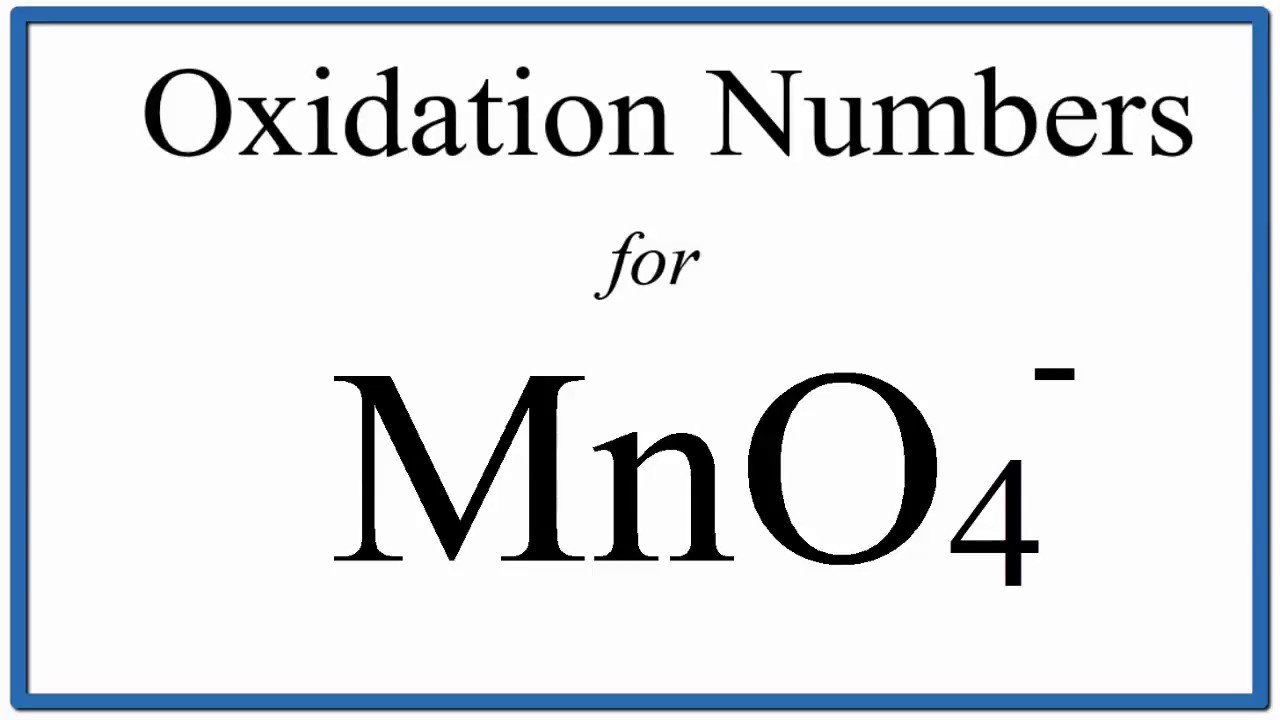
Ver How to find the Oxidation Number for Mn in the MnO4 – ion.
Discusión Balance the Redox Reaction for MnO4- + I- → I2 + Mn 2+ Último
Asunto Be(MNo4)2/ Permangato Berilico Camilo Arango Último
Oxidation number of mn in mno4 2- in Hindi | Chemistry by KclAcademy | ऑक्सीकरण अवस्था actualizar
Viral Redox Half Equation Example 1 (MnO4- to Mn2+) tendencias
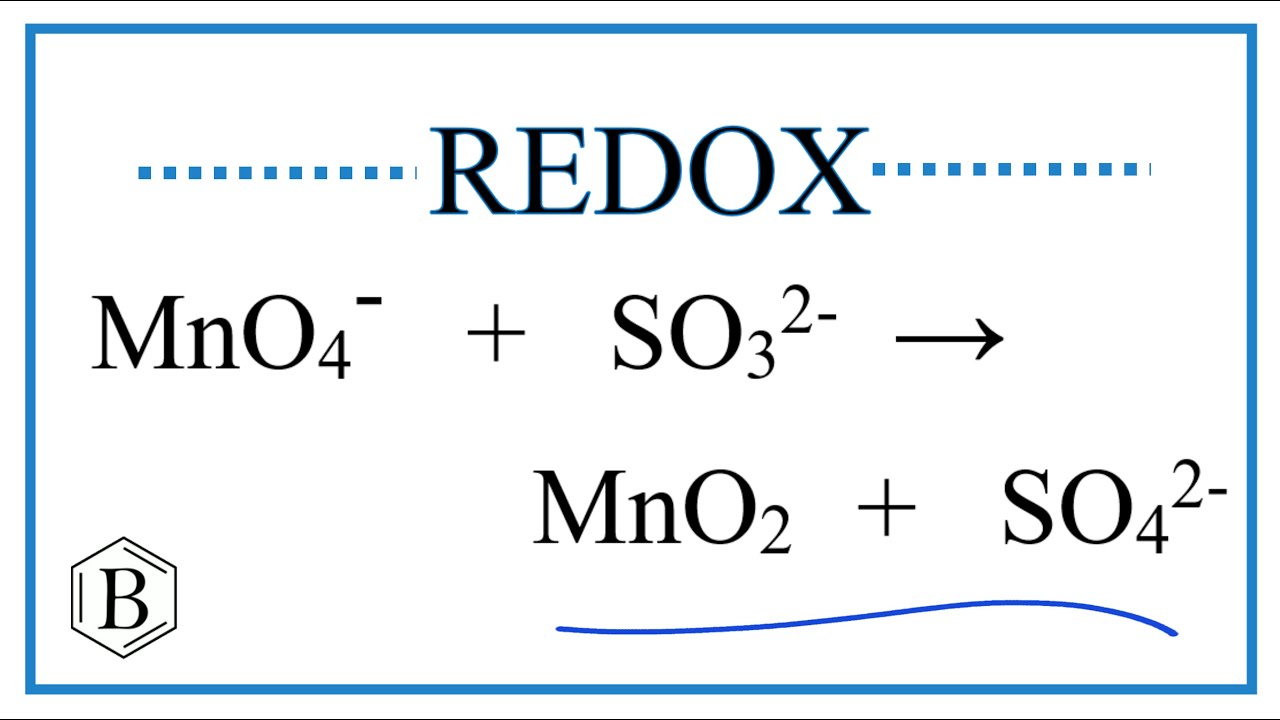
Viral Balance the Redox Reaction for MnO4- + SO3 2- → MnO2 + SO4 2- popular
Artículos 4.40e | Balance: H2O2(aq) + MnO4−(aq) → Mn2+(aq) + O2(g) (in acid) viral
Más sobre Be Mno4 2 de la discusión anterior
To find the correct oxidation state of Mn in Ca(MnO4)2 (Calcium permanganate ), and each element in the molecule, we use a few rules and some simple math.
First, since the Ca(MnO4)2 molecule doesn’t have an overall charge (like NO3- or H3O+) we could say that the total of the oxidation numbers for Ca(MnO4)2 will be zero since it is a neutral molecule.
We write the oxidation number (O.N.) for elements that we know and use these to figure out oxidation number for Mn.
———-
GENERAL RULES
Free elements have an oxidation state of zero (e.g. Na, Fe, H2, O2, S8).
In an ion the all Oxidation numbers must add up to the charge on the ion.
In a neutral compound all Oxidation Numbers must add up to zero.
Group 1 = +1
Group 2 = +2
Hydrogen with Non-Metals = +1
Hydrogen with Metals (or Boron) = -1
Fluorine = -1
Oxygen = -2 (except in H2O2 or with Fluorine)
Group 17(7A) = -1 except with Oxygen and other halogens lower in the group
———-
We know that Oxygen usually is -2 with a few exceptions. When Oxygen is in a peroxide, like H2O2 (Hydrogen peroxide), it has a charge of -1. When it is bonded to Fluorine (F) it has an oxidation number of +1.
Here it is bonded to Mn so the oxidation number on Oxygen is -2. Using this information we can figure out the oxidation number for the element Mn in Ca(MnO4)2.
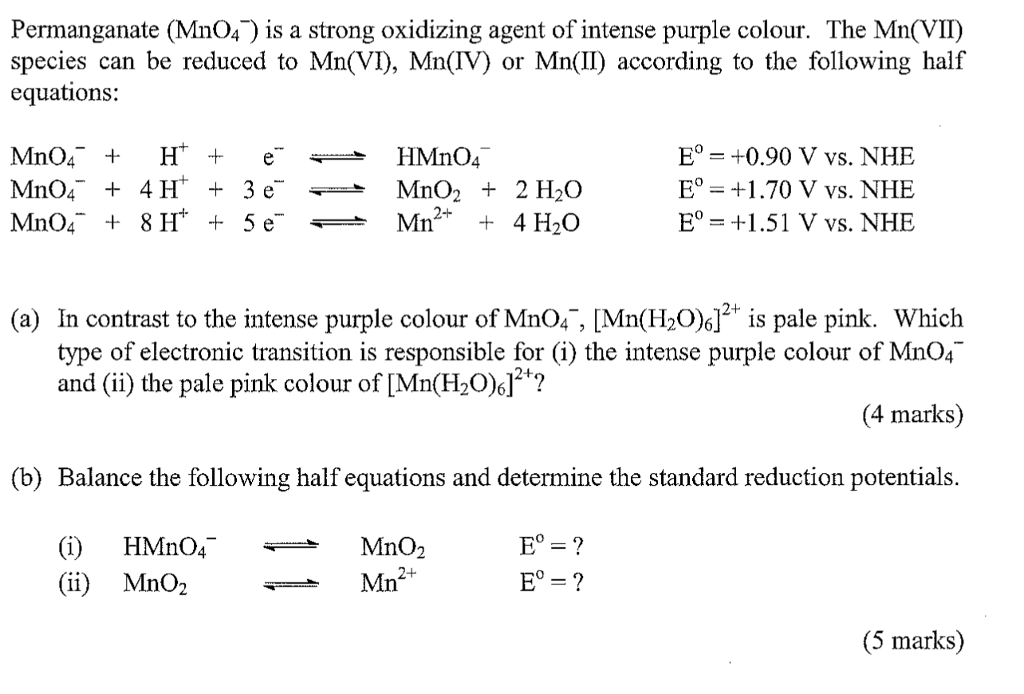
Discusión Solved: Permanganate (MnO4 ') Is A Strong Oxidizing Agent | Chegg.com actualizado

Asunto Balancing equation MnO4 – YouTube Último

Discusión Balance by half reaction. Mno4- + SO2. gives Mn2+ + HSO4- in acidic

Acerca de MnO4- + c2O42- _ MnO2+CO2 – Brainly.co.id

Acerca de Balancing Redox Equation with MnO4 – YouTube Último
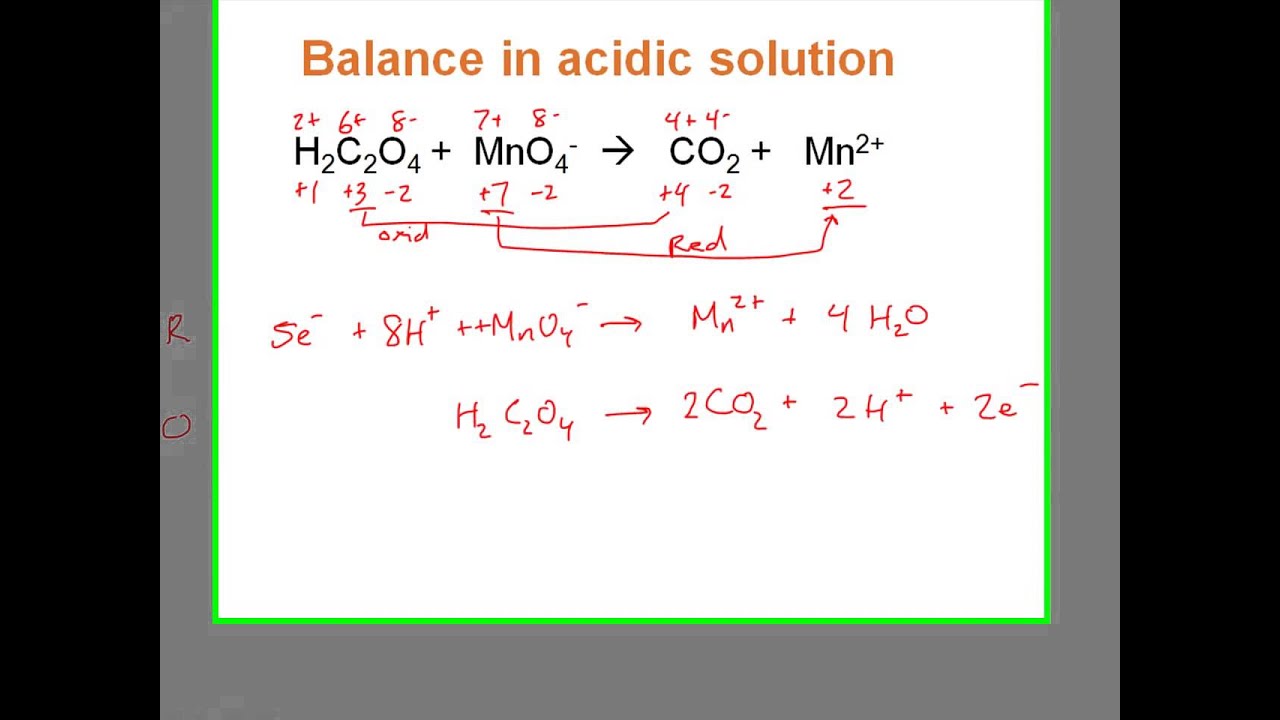
Asunto (a) Complete the following reactions in an aqueous medium: (i) MnO4 actualizar
Artículos PPT – Balancing Redox Equations PowerPoint Presentation, free download viral
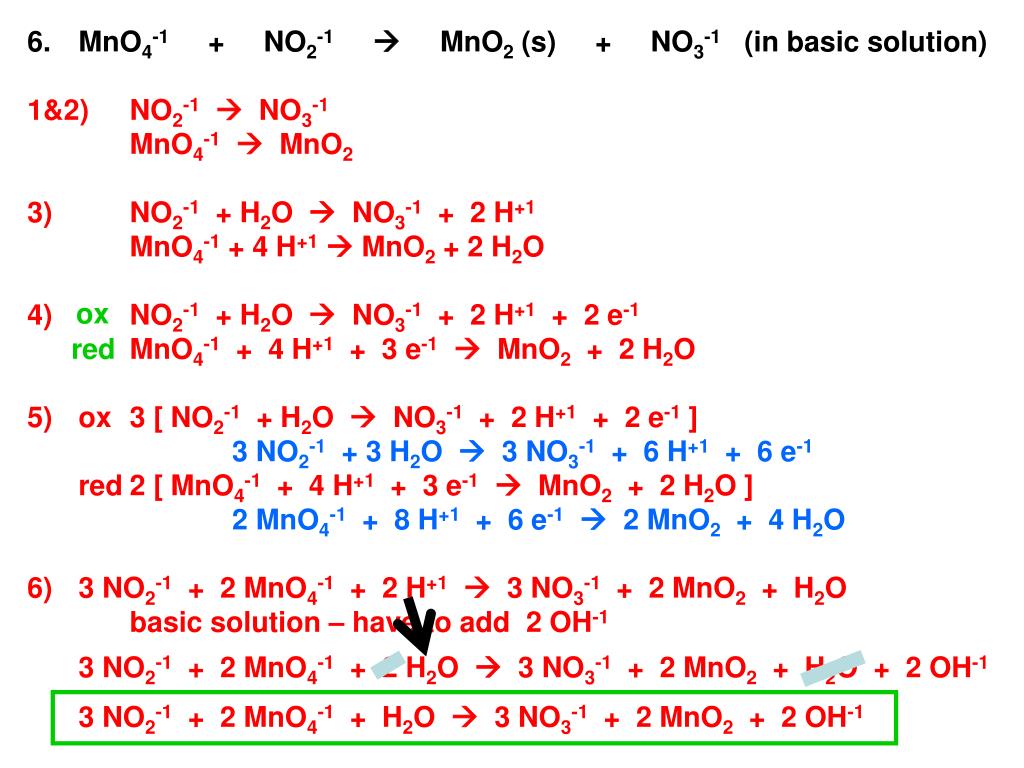
Ver Answered: 1.MnO4-(aq) + CH3OH (aq)… | bartleby Último
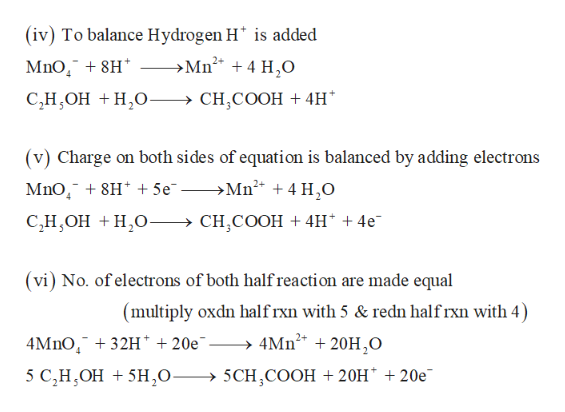
Temas 8H+ + MnO4- + 5e- Mn2+ + 4H2O × 2 2H2O + SO2 SO42- + 2e- + 4H+ × tendencias
.PNG)
Oxidation Number Of Mn In Kmno4 | Fun Practice and Test Último