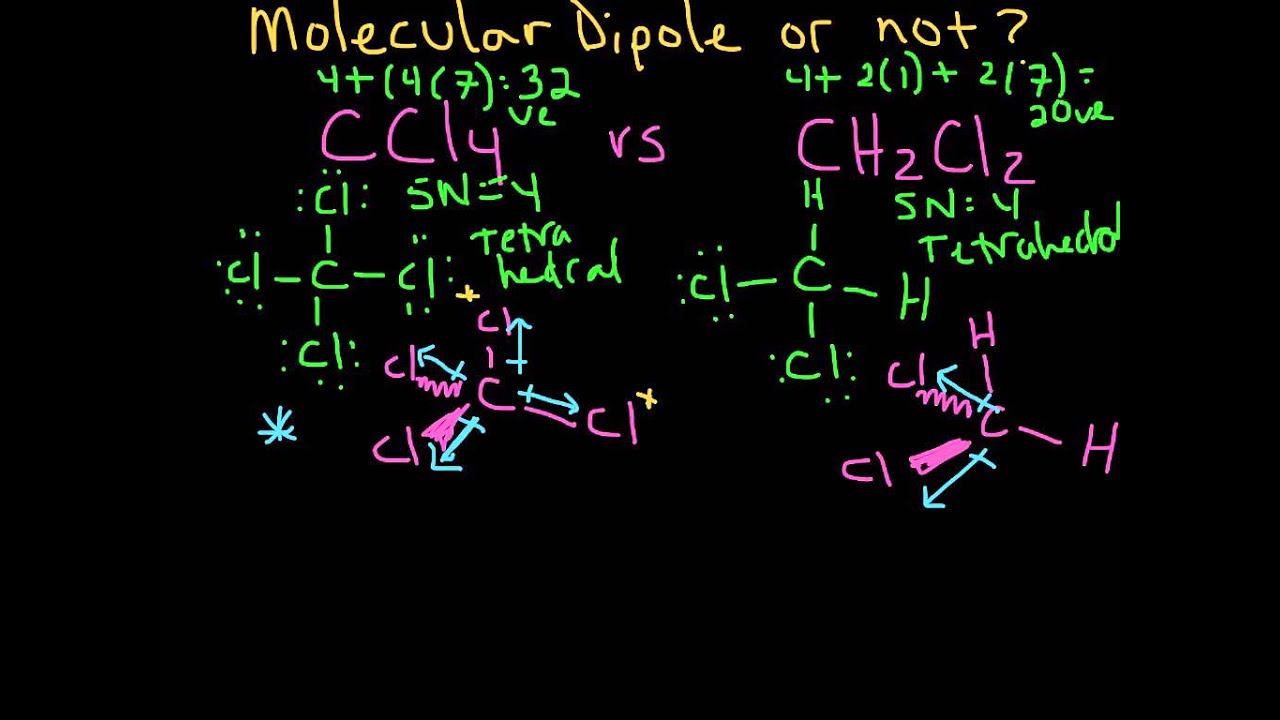
Estructura de lewis del chcl3 (cloroformo) anuncios adsense. Estructura de lewis del n2o (óxido nitroso) What is the structure lewis of (ch3) 2nh2cl? The compound is dimethylammonium chloride.
Frases De Juan Salvador Gaviota, 20.58 MB, FRASES INCREÍBLES DEL LIBRO ”JUAN SALVADOR GAVIOTA”✨ | RICHARD BACH | Nenfelay ⭐️, 14:59, 1,015, Nenfelay, 2021-02-24T18:00:14.000000Z, 19, Pin de Tayira Mora Black en Inspiraciòn en español | Juan salvador, www.pinterest.com.au, 960 x 720, jpeg, , 20, frases-de-juan-salvador-gaviota, Zadania lekcyjne
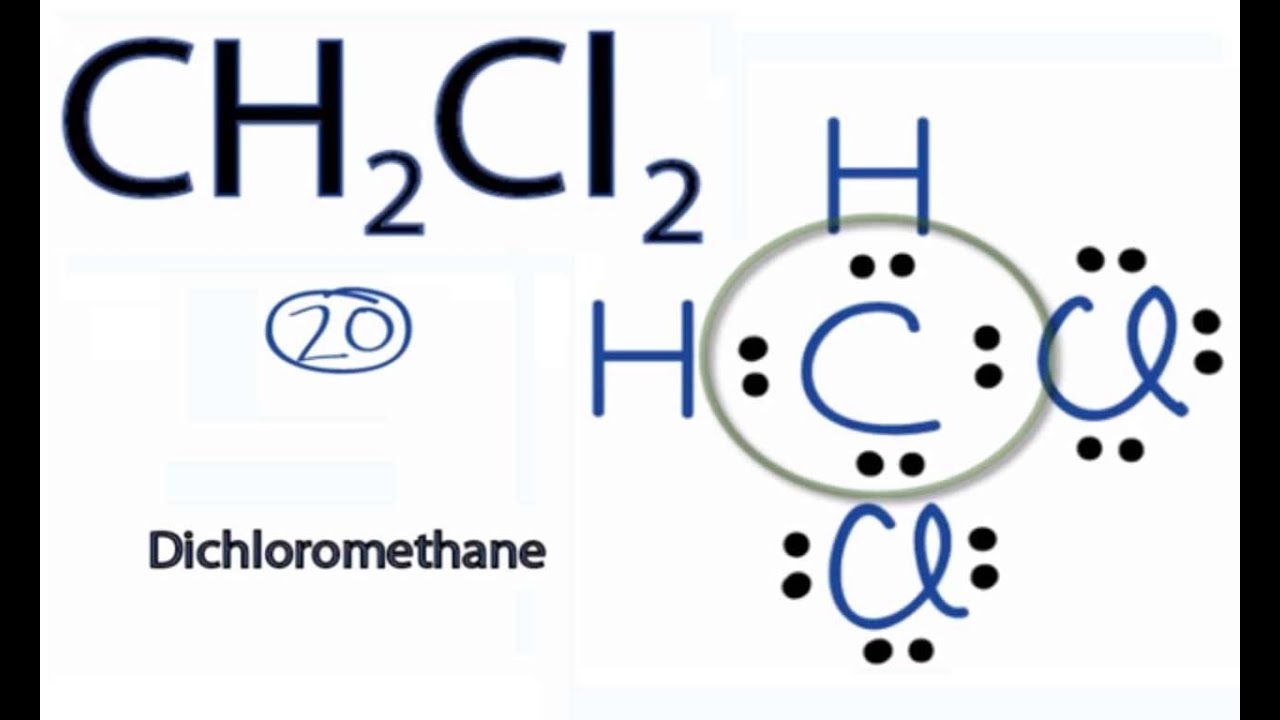
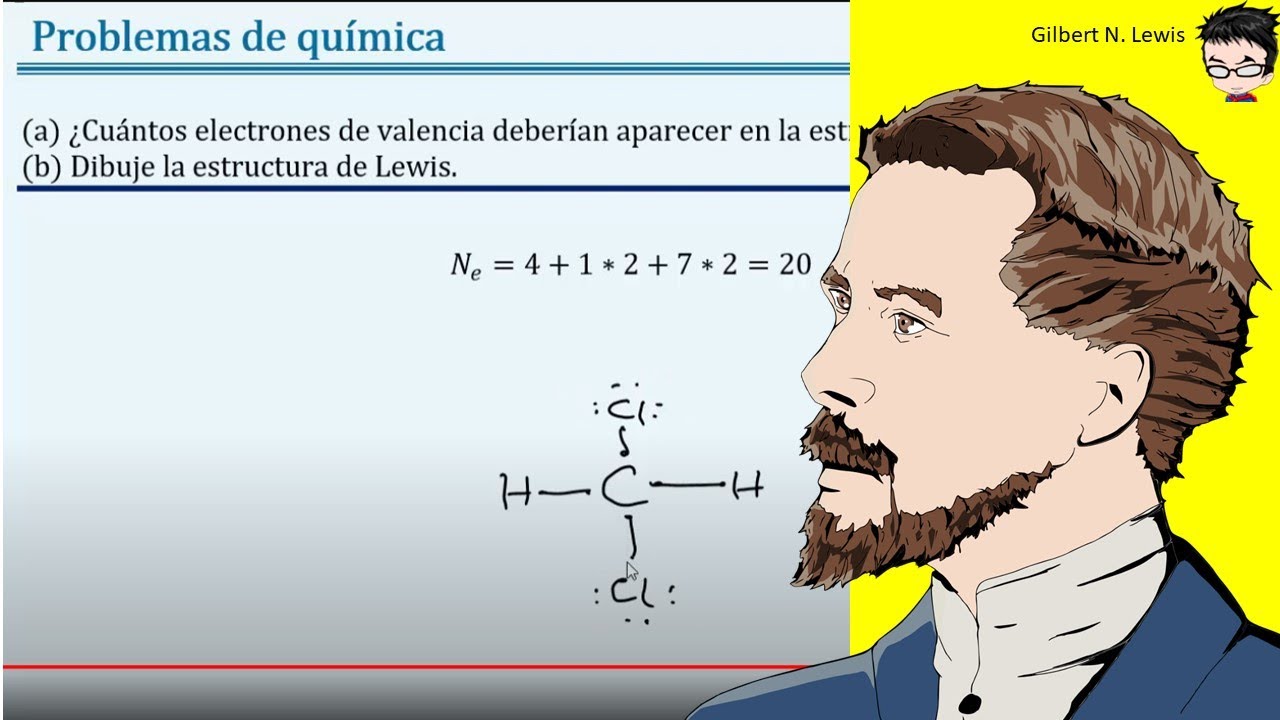
D'electrons de valència, n = no. En la estructura de lewis las líneas representan los enlaces y los puntos representan los electrones de valencia. Cuando hablamos de ch2cl2, el carbono es. Steps to draw a lewis structure of hypochlorite (clo) step 1: Determine the total number of valence electrons in one hypochlorite molecule. The chlorine atom has seven. Cuántos mol de nacio (hipoclorito de sodio) se obtendrán a partir de 25 mol de naoh2. Cuantos gramos de naci se obtendrán. Carbono tiene cuatro electrones sin emparejar.
Últimas CH2Cl2 Lewis Structure: How to Draw the Lewis Structure for CH2Cl2 (Dichloromethane) Nuevo
Viral Estructura De Lewis De Diclorometano (CH2Cl2)
Veamos 𝐃𝐢𝐛𝐮𝐣𝐚𝐫 la 𝐞𝐬𝐭𝐫𝐮𝐜𝐭𝐮𝐫𝐚 de 𝐋𝐞𝐰𝐢𝐬 para 𝐂𝐇𝟐𝐂𝐥𝟐
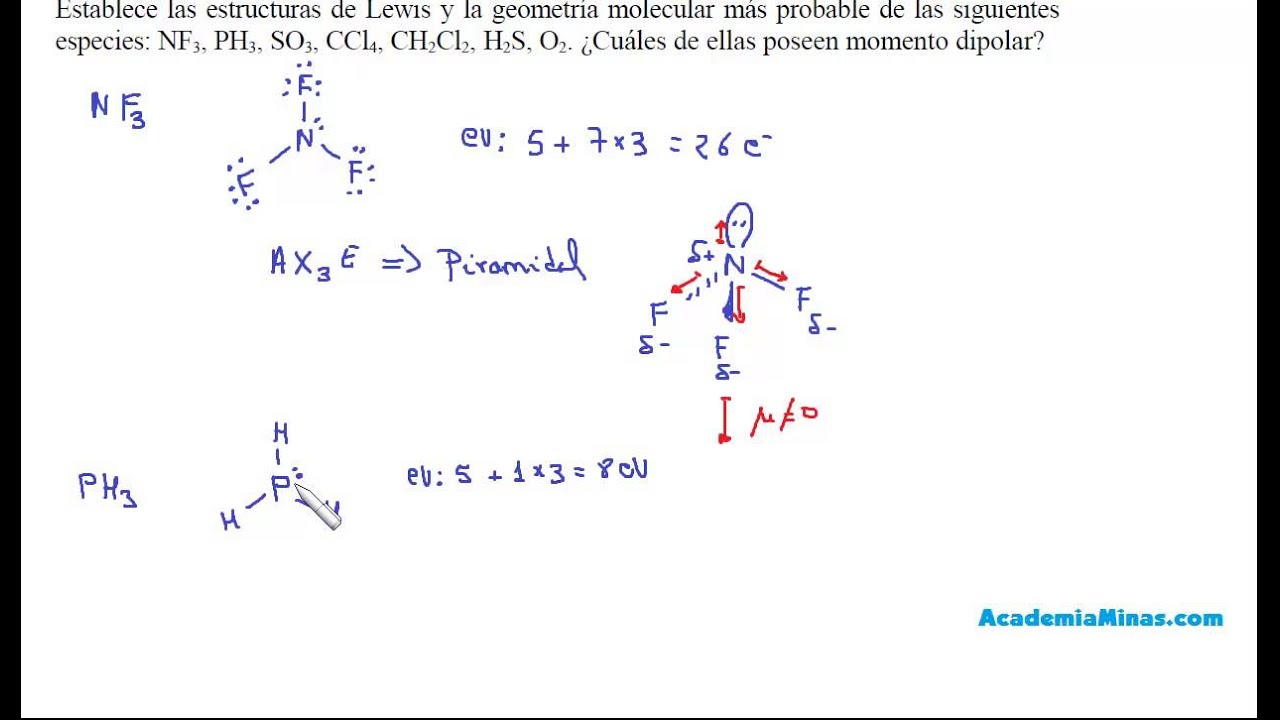
Últimas Estructuras de Lewis, geometría y polaridad de NF3, PH3, SO3, CCl4, CH2Cl2, H2S, O2 viral
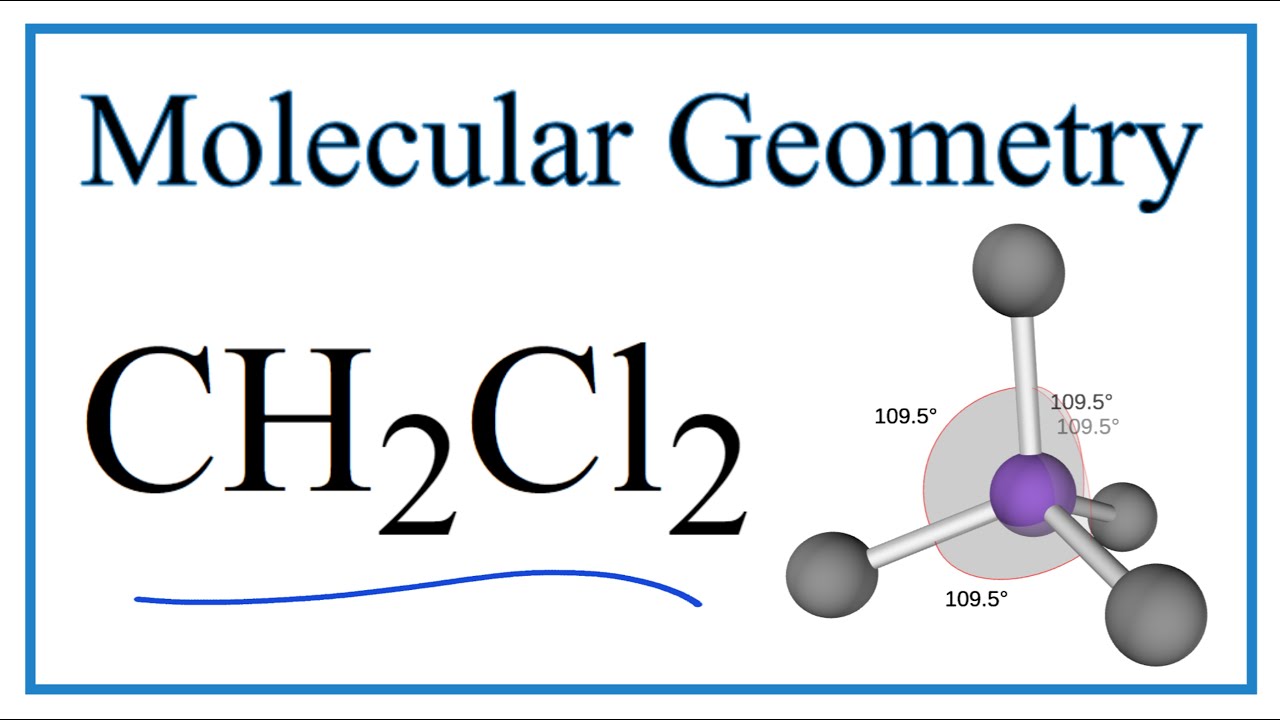
Acerca de CH2Cl2 Molecular Geometry, Bond Angles (and Electron Geometry) volviéndose viral
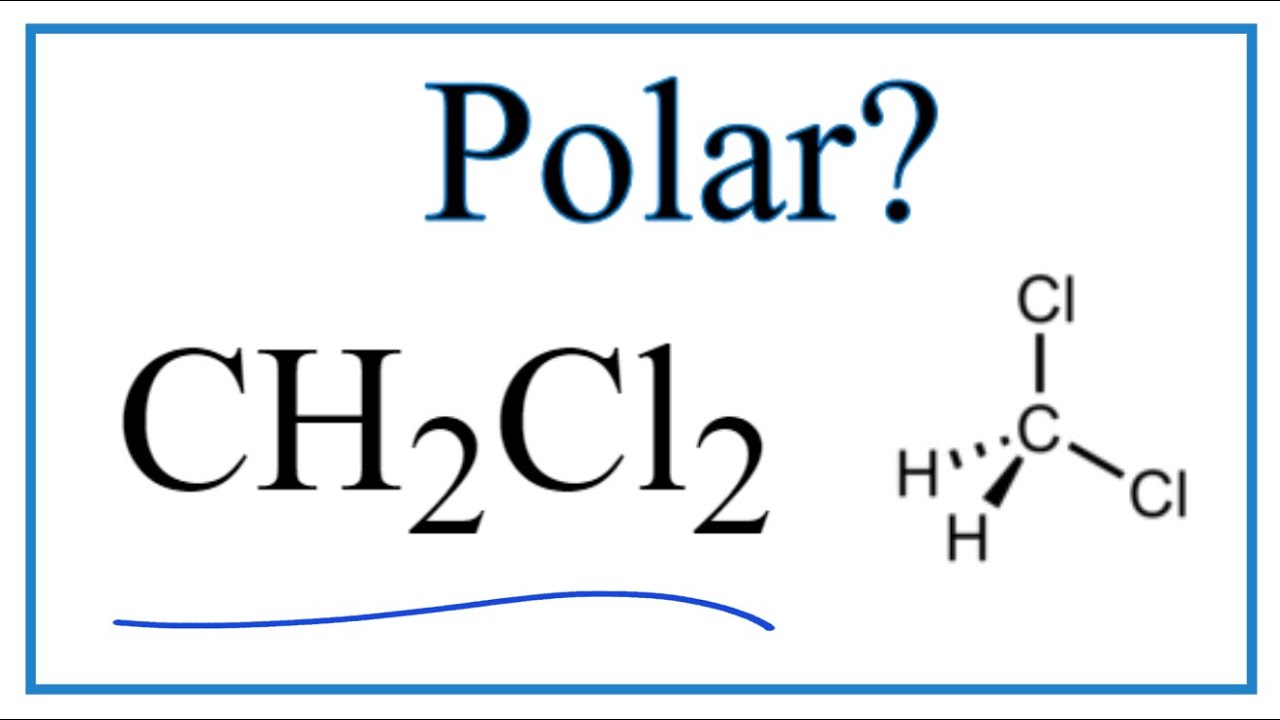
Actualmente – Is CH2Cl2 Polar or Nonpolar (Dichloromethane) actualizado
Noticias Draw the Lewis dot structure for CH2Cl2 Is this molecule polar tendencias
Chemistry : Chemical Bonding (VSEPR) and Lewis Structure for CH2Cl2 and SiF6(-2) Último
Temas Molecular Dipole Moment Example 2 (CCl4 and CH2Cl2) Último
Discusión Is dichloromethane CH2Cl2 polar or non-polar Explain
Detalles de Estructura De Lewis De Ch2cl2 que podría ser interesante
A step-by-step explanation of how to draw the CH2Cl2 Lewis Dot Structure (Dichloromethane).
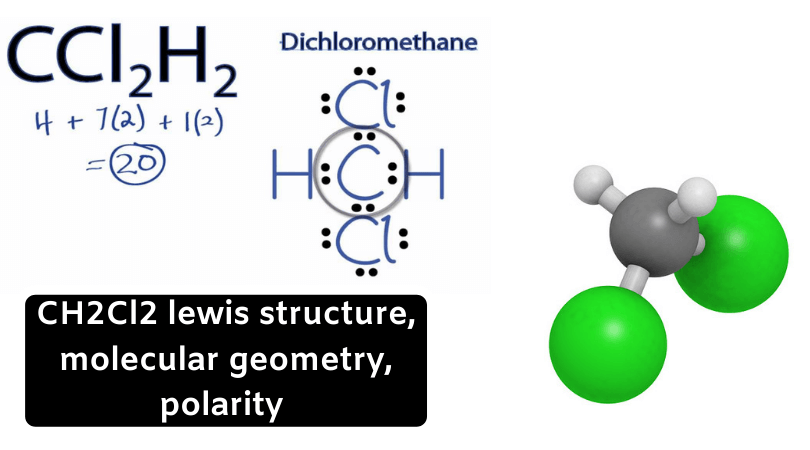
For the CH2Cl2 structure use the periodic table to find the total number of valence electrons for the CH2Cl2 molecule. Once we know how many valence electrons there are in CH2Cl2 we can distribute them around the central atom with the goal of filling the outer shells of each atom.
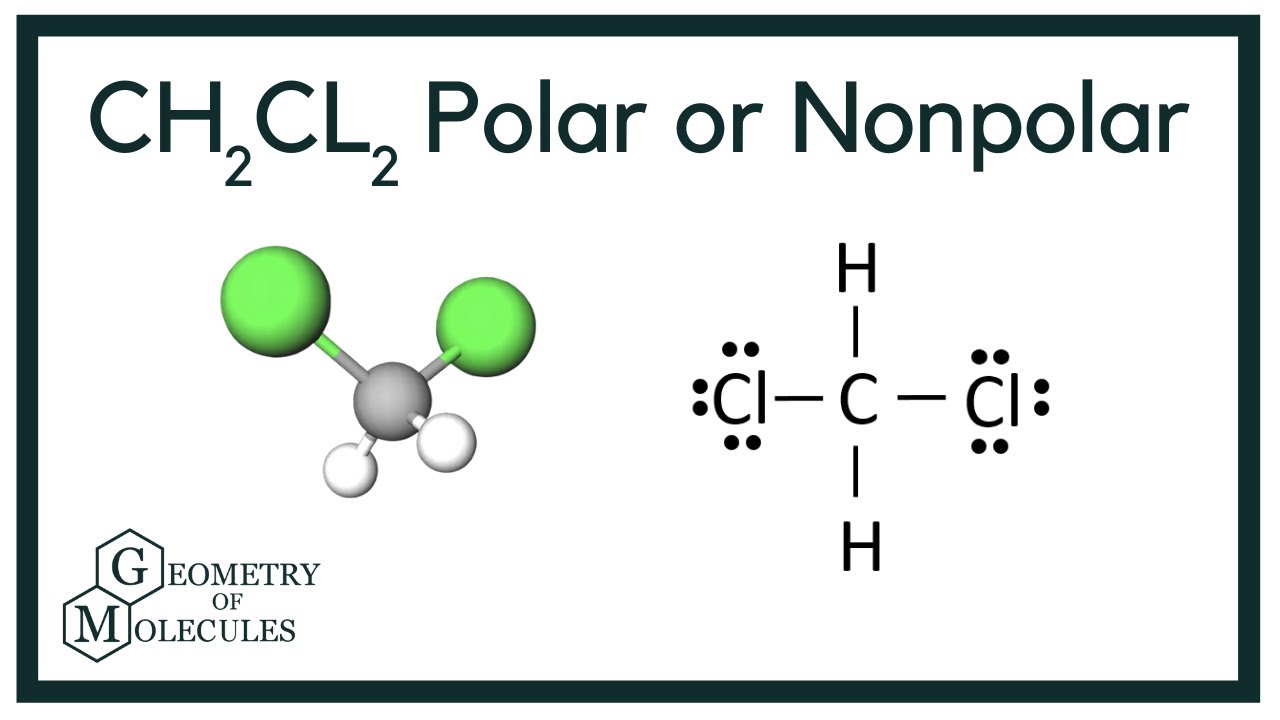
In the Lewis structure of CH2Cl2 structure there are a total of 20 valence electrons. CH2Cl2 is also called Dichloromethane.
—– Steps to Write Lewis Structure for compounds like CH2Cl2 —–
1. Find the total valence electrons for the CH2Cl2 molecule.
2. Put the least electronegative atom in the center. Note: Hydrogen (H) always goes outside.
3. Put two electrons between atoms to form a chemical bond.
4. Complete octets on outside atoms.
5. If central atom does not have an octet, move electrons from outer atoms to form double or triple bonds.
—– Lewis Resources —–
• Lewis Structures Made Simple: youtu.be/1ZlnzyHahvo
• More practice: youtu.be/DQclmBeIKTc
• Counting Valence Electrons: youtu.be/VBp7mKdcrDk
• Calculating Formal Charge: youtu.be/vOFAPlq4y_k
• Exceptions to the Octet Rule: youtu.be/Dkj-SMBLQzM
Lewis Structures, also called Electron Dot Structures, are important to learn because they help us understand how atoms and electrons are arranged in a molecule, such as Dichloromethane. This can help us determine the molecular geometry, how the molecule might react with other molecules, and some of the physical properties of the molecule (like boiling point and surface tension).
Chemistry help at Breslyn.org
Noticias Ch2cl2 Polar Or Nonpolar – learn.lif.co.id

Viral CH2Cl2 Lewis Structure: How to Draw the Lewis Structure for CH2Cl2 viral

Imágenes CH2Cl2 lewis structure, molecular geometry, polarity | Dichloromethane popular
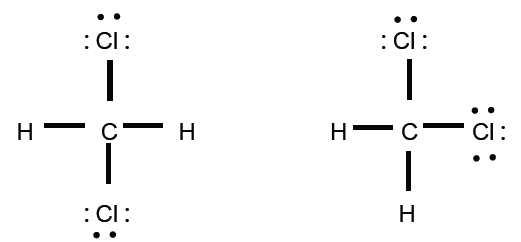
5. Consider methylene chloride (CH2Cl2). Two | Chegg.com
Noticias Estructuras de Lewis, geometría y polaridad de NF3, PH3, SO3, CCl4 viral

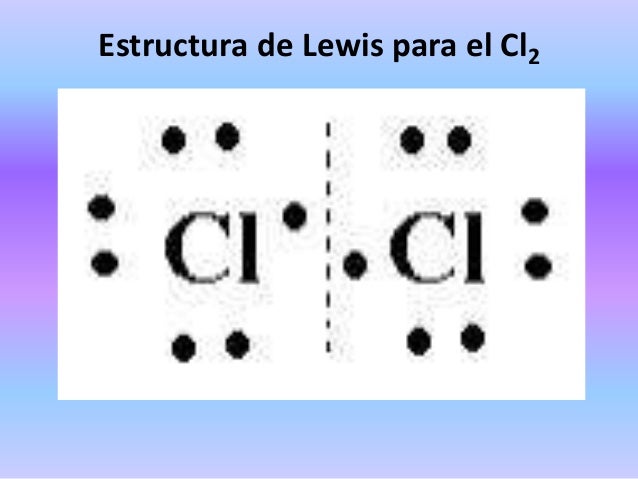
Imprescindible Estructura De Lewis Be Cl2 – 2021 idea e inspiración popular
Noticias CHCl3 Lewis Structure: How to Draw the Lewis Structure for CHCl3 – YouTube tendencias

Último Estructura De Lewis Be Cl2 – 2021 idea e inspiración actualizado

Apresente a estrutura de Lewis para os compostos a seguir e proponha a

estructura de lewis de CH2O – Brainly.lat