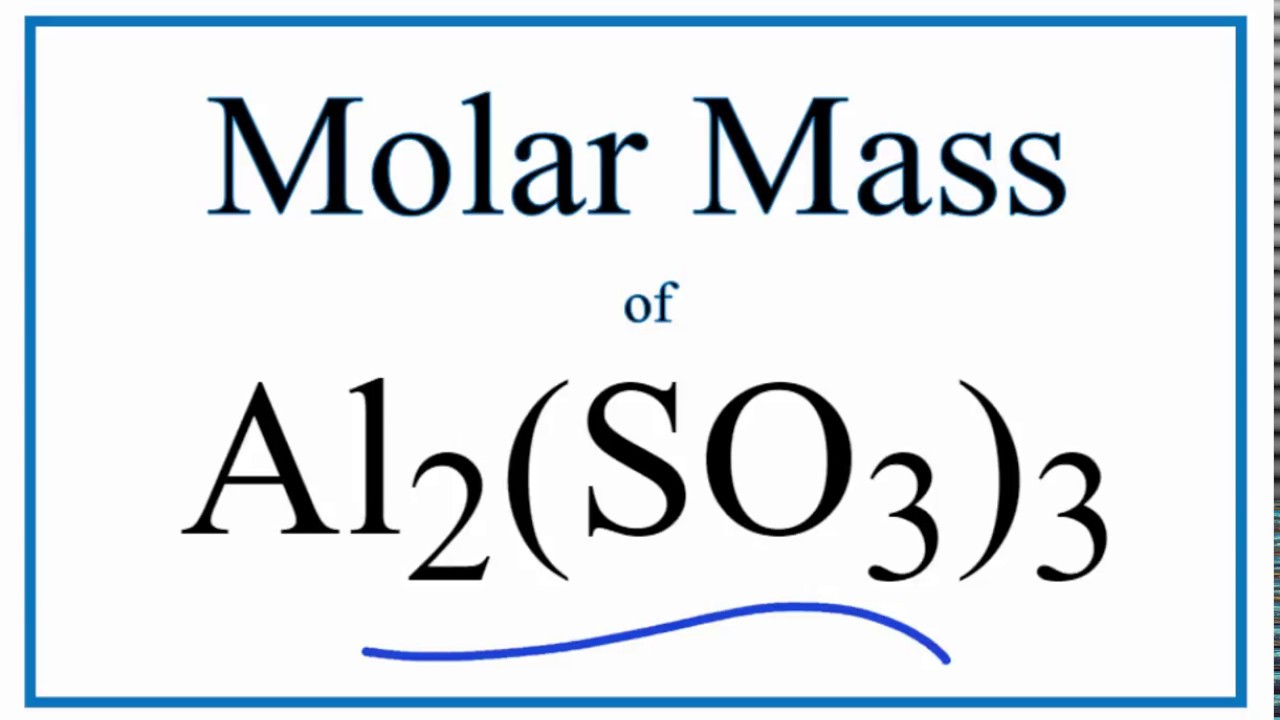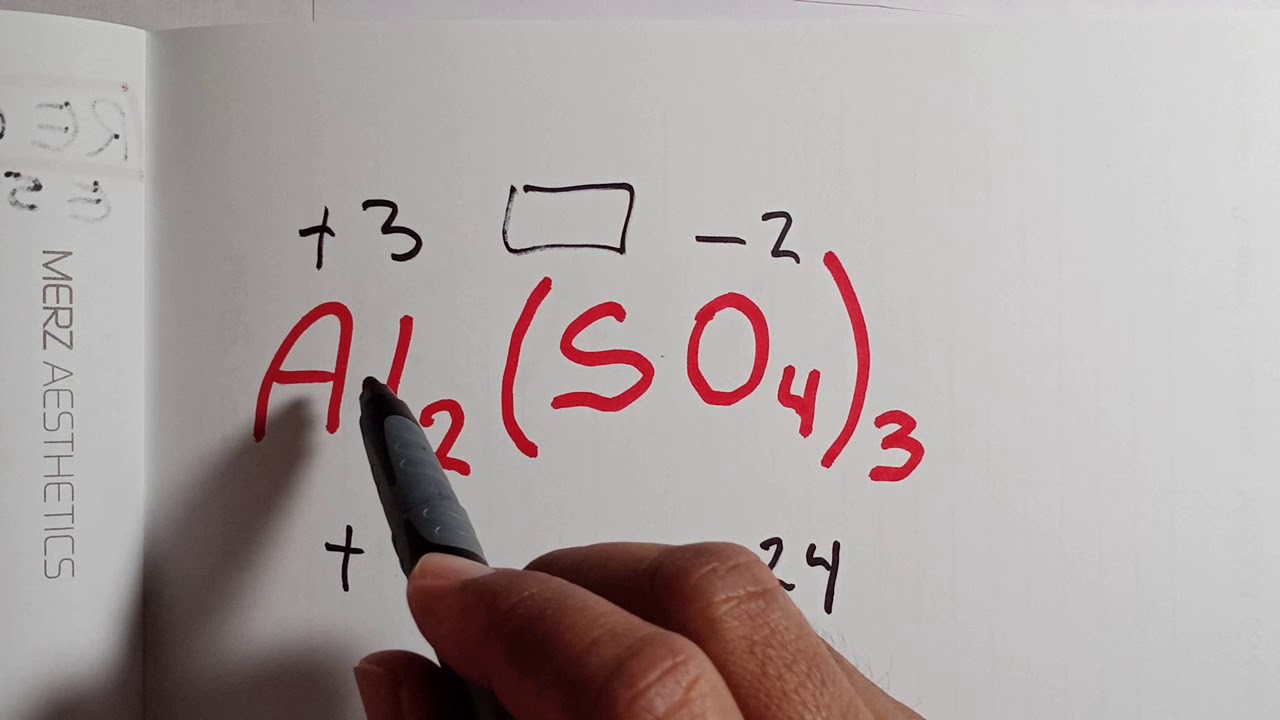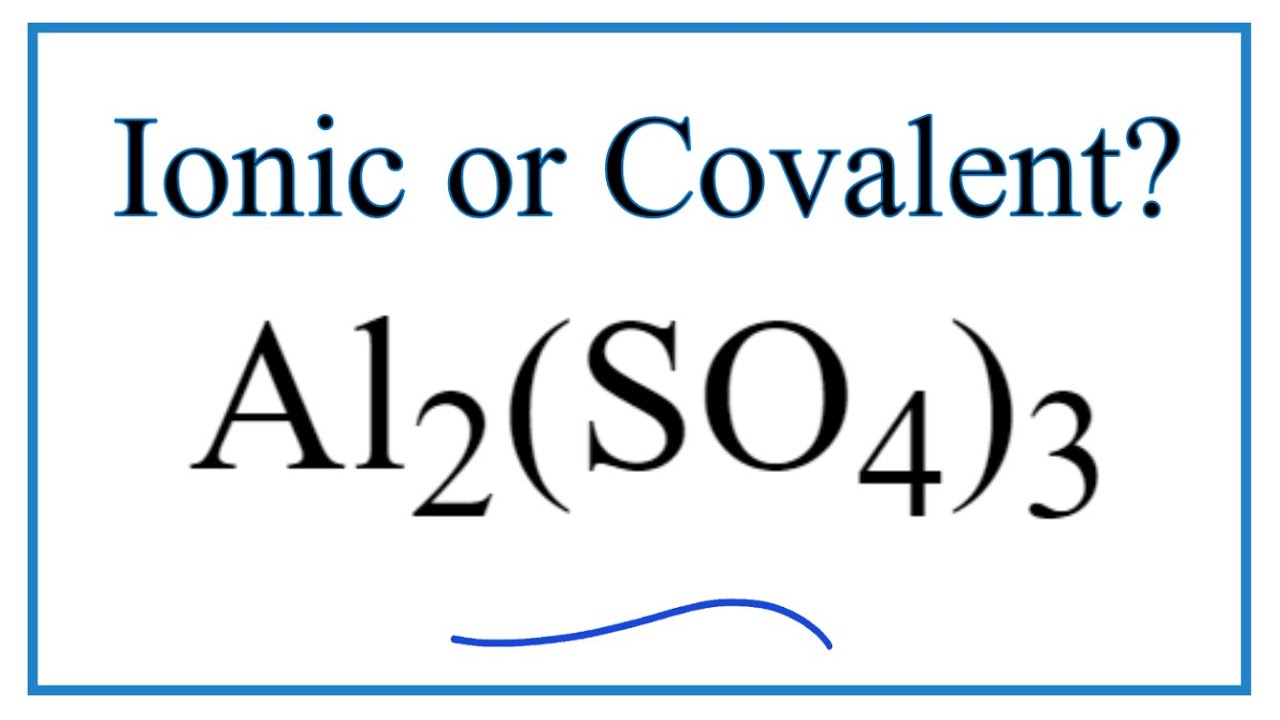Reacción que produce la sustancia al2 (sio3) 3 (nhôm silicat khan) () no se encontró reacción reacción que produce la sustancia al2o3 (nhôm oxit) (óxido de aluminio) 4al + 3o 2 → 2al 2 o 3 2al + 3cuo → al 2 o 3 + 3cu 2al + fe 2 o 3 → al 2 o 3 + 2fe reacción que produce la sustancia cr2 (sio3) 3 (crom (iii) silicato) (cromo (iii) silicato) Haz clic aquí 👆 para obtener una respuesta a tu pregunta ️ estado de oxidación de al2(sio3)3 por favor necesito ayda con todos los pasos. Formulación y nomenclatura de al2(so3)3, tris[trioxosulfato (iv)] de dialuminio, sulfito de aluminio, sulfito alumínico | formulacionquimica. com Al2(sio3)3 | chemical substance.
Ejemplos De Suma Iterada, 10.71 MB, Suma iterada, 07:48, 48,606, Ayudantia VSM, 2020-08-15T20:37:32.000000Z, 19, Suma iterada Ficha interactiva, es.liveworksheets.com, 1000 x 1413, jpeg, suma iterada liveworksheets interactiva, 20, ejemplos-de-suma-iterada, Zadania lekcyjne
Al 2 (sio 3) 3 short form al 2 o 9 si 3 nhôm silicat khan atomic_weight (g/mol) 282. 2142. Reaction that produces substance al2 (sio3)3 (nhôm silicat khan) () no reaction found reaction that produces substance al2o3 (nhôm oxit) (aluminium oxide) 4al + 3o 2 → 2al 2 o 3 2al + fe 2 o 3 → al 2 o 3 + 2fe 2al + 3cuo → al 2 o 3 + 3cu reaction that produces substance cr2 (sio3)3 (crom (iii) silicat) (chromium (iii) silicate) Aluminum metasilicate al2(sio3)3 molar mass, molecular weight 與反應物 al2(sio3)3 的平衡化學反應方程式 | 找到 1 個化學方程式 Formule in hill systeem is al2o9si3: Berekent de molaire massa (molaire gewicht) to calculate molar mass of a chemical compound enter its formula and click 'compute'. Balanced chemical reaction equations nema reactant al2 (sio3) 3 | 1 makemikari equation akawanikwa Na+ = sodium mg2+ = magnesium al3+ = aluminum 2) find the polyatomic ion on the. Aluminium silicate aluminium silicate (or aluminum silicate) is a name commonly applied to chemical compounds which are derived from aluminium oxide, al 2 o 3 and silicon dioxide, sio 2 which may be anhydrous or hydrated, naturally occurring as minerals or synthetic.
Ver How to Find the Number of Atoms in Al2(SO3)3 (Aluminum sulfite) viral
Acerca de Molar Mass / Molecular Weight of Al2(SO3)3: Aluminum Sulfite tendencias
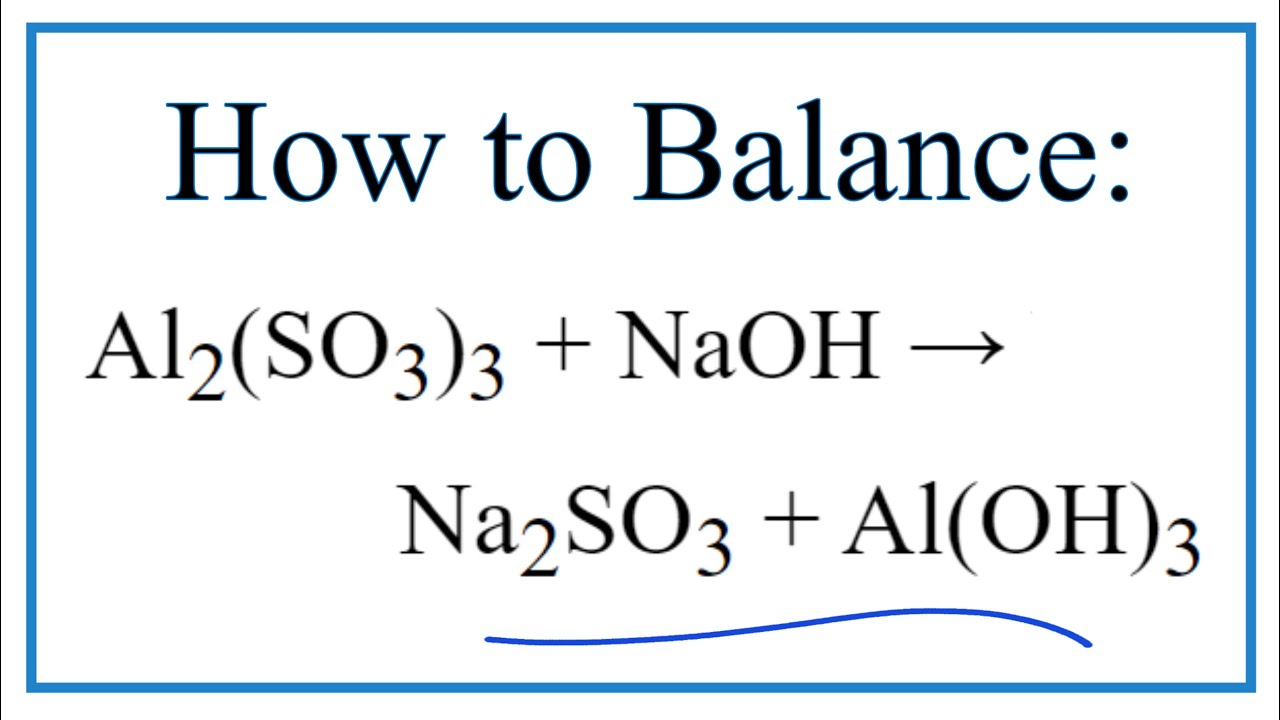
Mirar How to Balance Al2(SO3)3 + NaOH = Na2SO3 + Al(OH)3 Último
Viral How to Find the Number of Atoms in Al2(SO4)3 actualizado
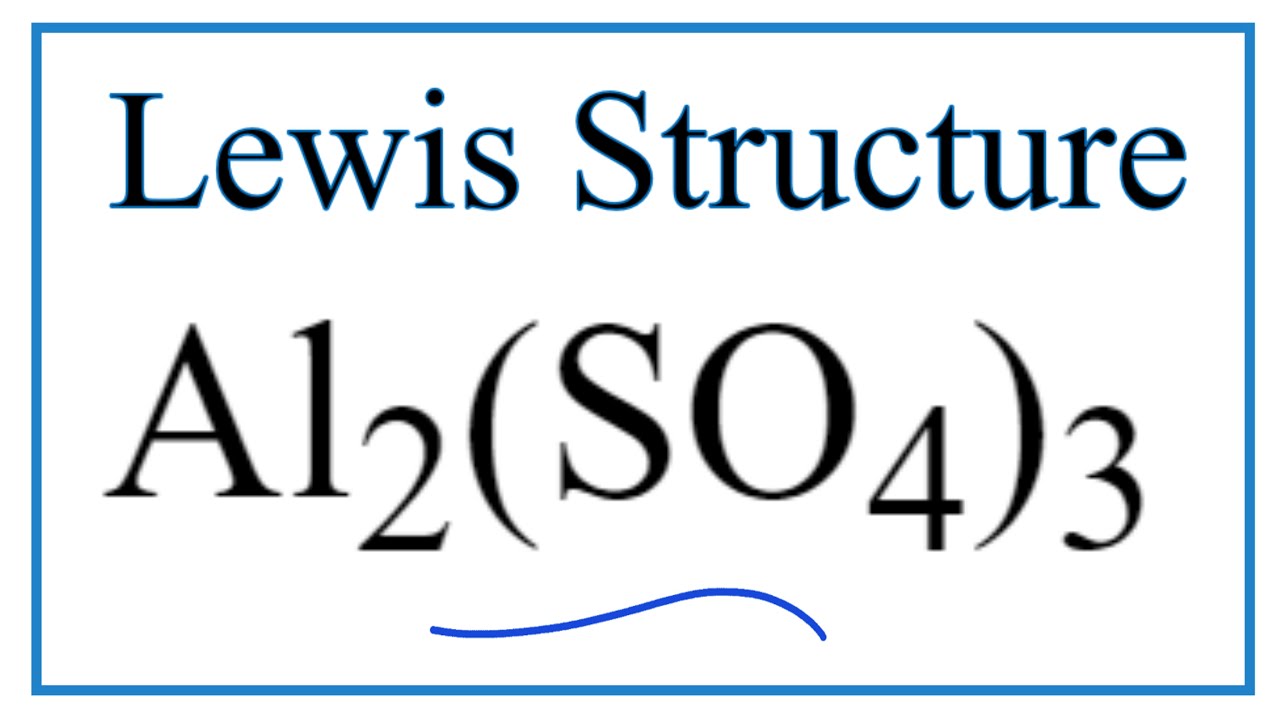
How to Draw the Lewis Dot Structure for Al2(SO4)3 viral
Actualmente – How to Find the Percent Composition by Mass for Al2(SO4)3 (Aluminum sulfate) Último
Videos OBTENCIÓN DEL NUMERO DE OXIDACIÓN tendencias
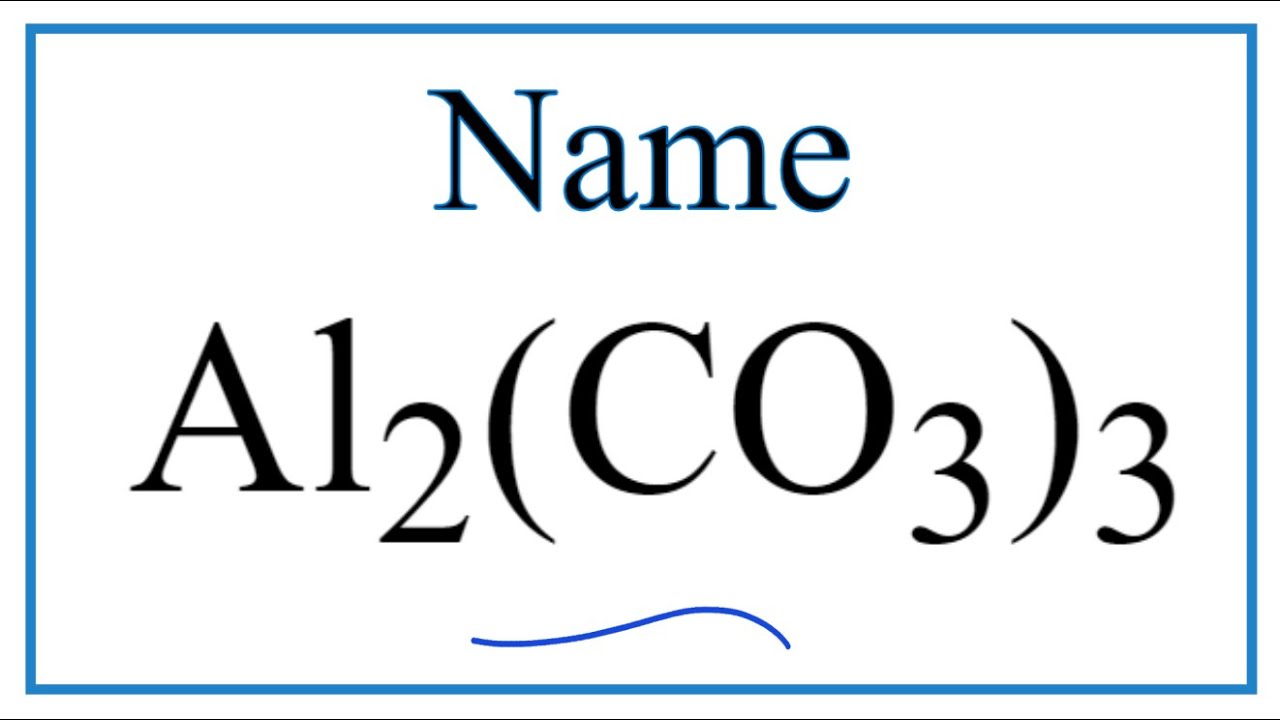
Últimas How to Write the Name for Al2(CO3)3 actualizar
Asunto Is Al2(SO4)3 (Aluminum sulfate) Ionic or Covalent Último
Actualmente – Beryl Be3Al2(SiO3)6 (Al light blue, Be green, Si dark blue) Último
Al2 Sio3 3 último
To find the total number of atoms in Al2(SO3)3 (Aluminum sulfite) we’ll add up the number of each type of atom. The small number after the element symbol is called the subscript and this tells us how many of that atom are in the compound. For example, H2 means there are two Hydrogen atoms.
When there is no number after an element, we assume there to be one atom of that element. For example, in H2O we have only one Oxygen atom since there isn’t a number after the O.
If there is a number in front of the compound (it’s called a “coefficient”), like 2H2O that means we have two separate molecules. We therefore we multiple the subscript for each element by the number in front. For 2H2O we have four H atoms (the coefficient 2 times the subscript 2) and two O atoms.
For compounds with parenthesis, like Ca(NO3)2, we multiple the atoms inside the parentheses by the subscript. In this case we would have two N atoms and six O atoms. There is only one Ca atom.
Finally, if you are asked to find the number of atoms in one mole, for example, the number of H atoms in one mole of H2O, you multiply the number of atoms by Avogadro’s number. In this case we have two H atoms so we multiply 2 x 6.02 x 10^23.
Mira Презентація до уроку на тему :" Загальна характеристика солей" actualizar

Temas PPT – В схеме превращений Ca X 1 X 2 PowerPoint Presentation – ID:3658610 tendencias

Artículos PPT – В схеме превращений Ca X 1 X 2 PowerPoint Presentation – ID:3658610 tendencias

Ver Написать уравнения диссоциации следующих соединений: KOH, H2SiO3, Al2 actualizar

Actualmente – estado de oxidación de Al2(SiO3)3 por favor necesito ayda con todos los popular

PPT – В схеме превращений Ca X 1 X 2 PowerPoint Presentation – ID:3658610

Noticias Презентация на тему: "Электролитическая диссоциация веществ Реакции actualizar

Mira Презентація на тему Поширення оксидів у оболонках Землі — презентації з más

Imágenes Ответы Mail.ru: Al2S3 – X1 – X2 – KAlO2 a)Al2O3 и AlCl3 б) Al2(SiO3)3 и volviéndose viral

Ver Ответы Mail.ru: 3Na2SiO3+2AlCl3=6NaCl+Al2(SiO3)3 написать ионное уравнение viral