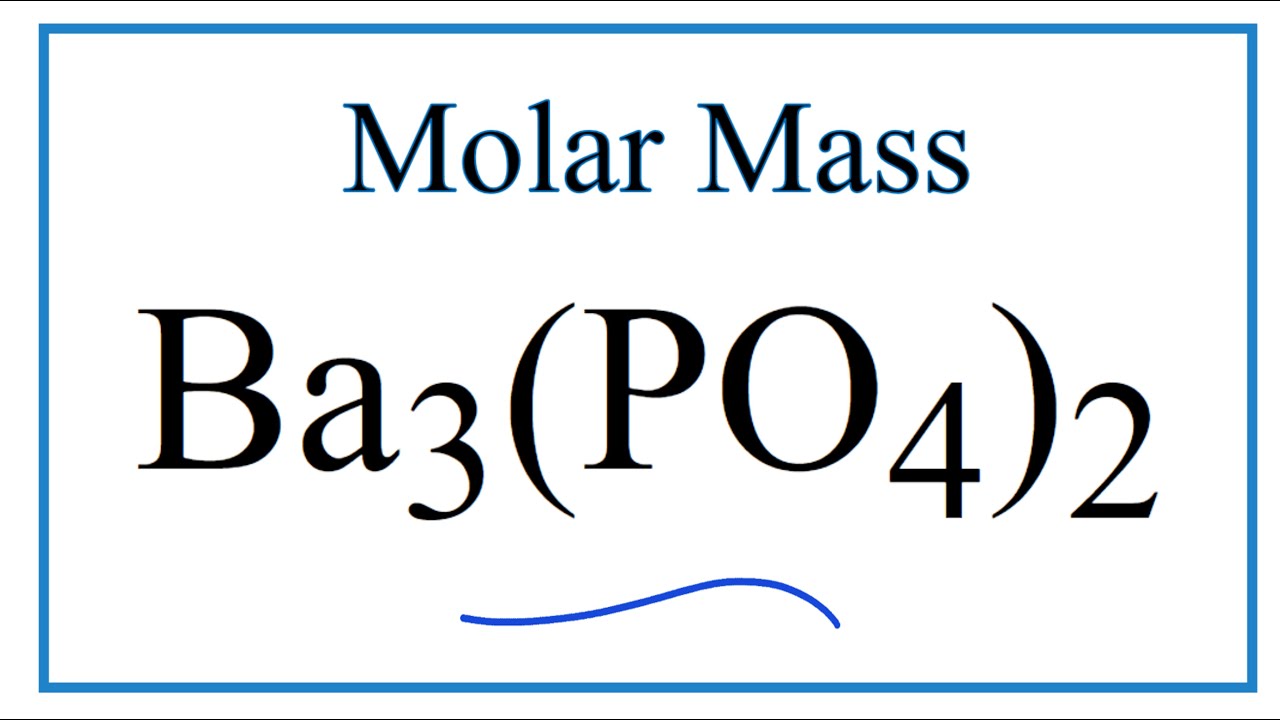Encontre uma resposta para sua pergunta uma solução 0,1 molar de al4 (p2o7)3 está com densidade de 0,63 g/ml. qual sua porcentagem em massa ?? Molar mass of al4 (p2o7)3 is 629. 7561 g/mol get control of 2022! Track your food intake, exercise, sleep and meditation for free. Convert between al4 (p2o7)3 weight and moles.
50 Psi A Bar, 5.24 MB, BAR, PSI, Pressure Measurement EXPLAINED, 03:49, 31,261, SpeedLab Channel, 2020-07-27T05:43:34.000000Z, 19, Pressure Gauge 50mm Dial 0/30 PSI & 0/2 Bar 1/4 BSPT BOTTOM and/or Hose, www.ebay.co.uk, 805 x 1000, jpeg, pressure gauge psi bar 50mm bspt dial bottom tails hose, 20, 50-psi-a-bar, Zadania lekcyjne
Once you know how many of each type of atom. モル質量 of al4(p2o7)3 is 629. 7561 g/mol. Convert between al4(p2o7)3 weight and moles. Al(no3)3 reacts with na4p2o7 to produce al4(p2o7)3 according to the equation 4al(no3)3 (aq) + 3na4p2o7 (aq) →. Heptaoxodifosfato (v) de aluminio ¿qué son sales neutras? El anión procede de un ácido que ha perdido todos sus hidrógenos; Pueden formarse las sales. 摩尔质量 of al4(p2o7)3 is 629. 7561 g/mol. Convert between al4(p2o7)3 weight and moles.
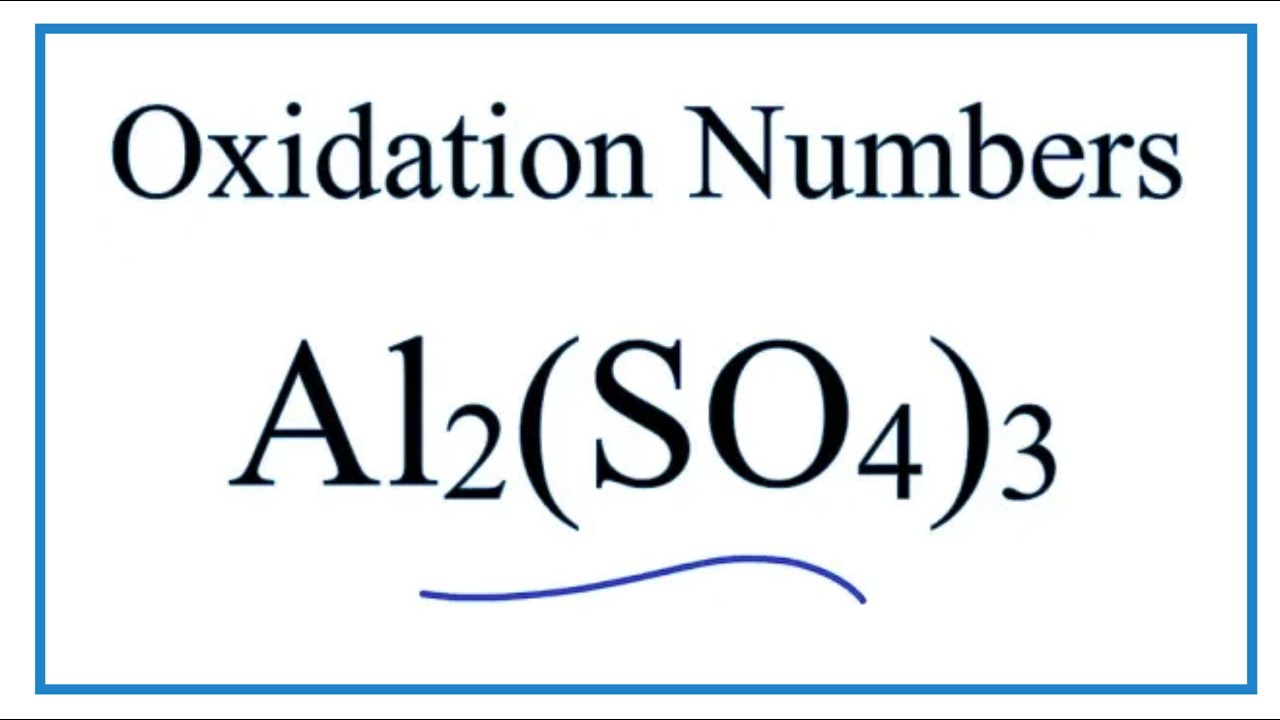
Ver How to find the Oxidation Number for Al in Al2(SO4)3 (Aluminum sulfate)
Ver QUIMICA Estructura de Lewis Ácido Pirofosfórico H4P2O7 Carga Formal AULAEXPRESS
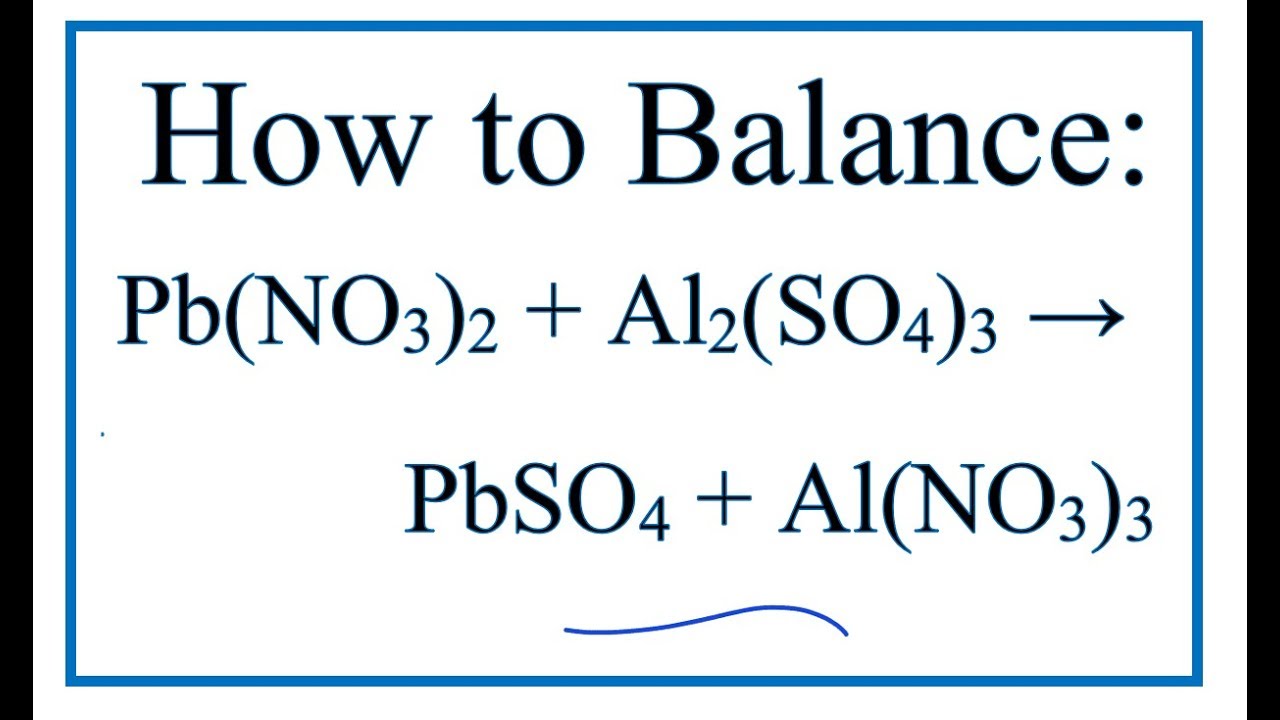
Aquí How to Balance Pb(NO3)2 + Al2(SO4)3 = PbSO4 + Al(NO3)3 más
Ver Molar Mass / Molecular Weight of Ba3(PO4)2: Barium phosphate más
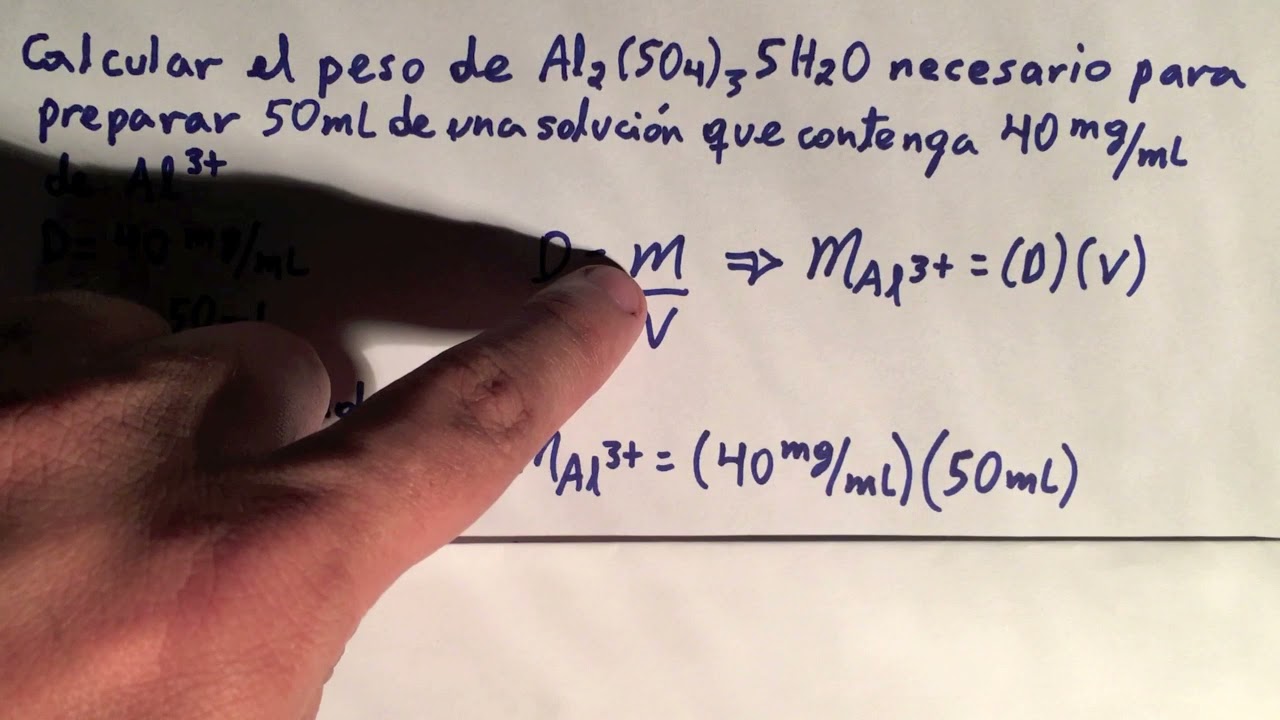
Viral Calcular la masa de Al2(SO4)3 necesaria para preparar 50ml de solucion Al3+ con densidad 40mg/mL viral
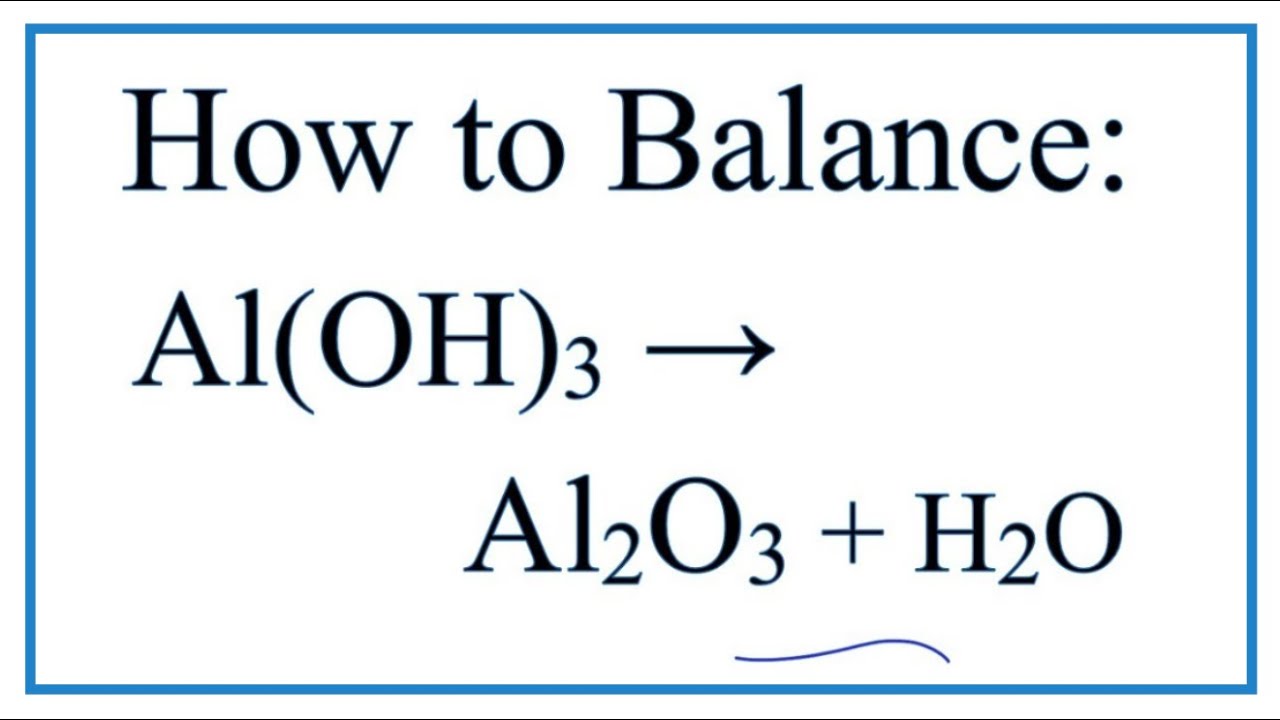
Obligatorio How to Balance Al(OH)3 = Al2O3 + H2O (at high temperatures) actualizar
Viral Como fazer Balanceamento de Equações Químicas popular
Nuevo Calcule el número de gramos de Al en 371 g de Al2O3
Acerca de ✅ECUACIONES por MÉTODO ALGEBRAICO | MÉTODO 100% EFECTIVO| QUÍMICA actualizar
Como calcular a massa molecular – (Massa das moléculas)
Al4 P2o7 3 que podría ser interesante
To find the correct oxidation state of Al in Al2(SO4)3 (Aluminum sulfate), and each element in the molecule, we use a few rules and some simple math.
First, since the Al2(SO4)3 molecule doesn’t have an overall charge (like NO3- or H3O+) we could say that the total of the oxidation numbers for Al2(SO4)3 will be zero since it is a neutral molecule.
We write the oxidation number (O.N.) for elements that we know and use these to figure out oxidation number for Al.
———-
GENERAL RULES
Free elements have an oxidation state of zero (e.g. Na, Fe, H2, O2, S8).
In an ion the all Oxidation numbers must add up to the charge on the ion.
In a neutral compound all Oxidation Numbers must add up to zero.
Group 1 = +1
Group 2 = +2
Hydrogen with Non-Metals = +1
Hydrogen with Metals (or Boron) = -1
Fluorine = -1
Oxygen = -2 (except in H2O2 or with Fluorine)
Group 17(7A) = -1 except with Oxygen and other halogens lower in the group
———-
We know that Oxygen usually is -2 with a few exceptions. When Oxygen is in a peroxide, like H2O2 (Hydrogen peroxide), it has a charge of -1. When it is bonded to Fluorine (F) it has an oxidation number of +1.
Here it is bonded to Al so the oxidation number on Oxygen is -2. Using this information we can figure out the oxidation number for the element Al in Al2(SO4)3.
Fotos Помогите с химией. Нужно образовать формулу соли – Школьные Знания.com más

Acerca de Qual é o nox do Al em Al4(P2O7)3? – Socorram-me, Recs! 2ª Série actualizado

PPT – KWASY NIEORGANICZNE PowerPoint Presentation, free download – ID
Imágenes PPT – KWASY NIEORGANICZNE PowerPoint Presentation, free download – ID Último
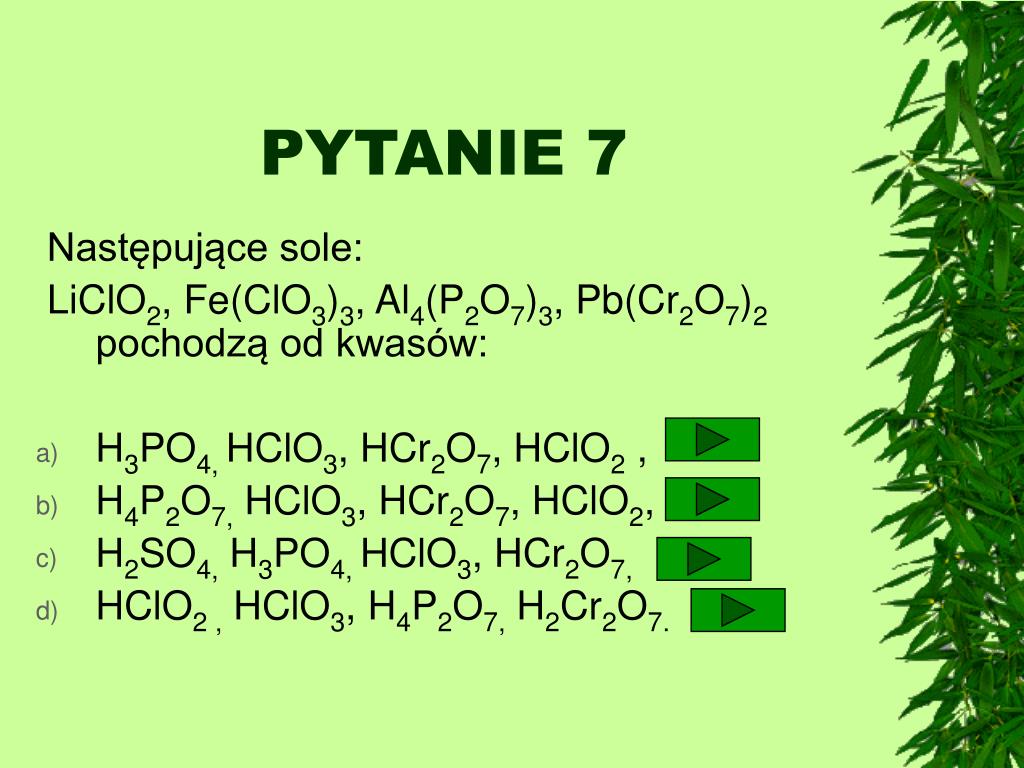
Nuevo PPT – KWASY NIEORGANICZNE PowerPoint Presentation, free download – ID

Imprescindible PPT – KWASY NIEORGANICZNE PowerPoint Presentation, free download – ID actualizado

Mira PPT – KWASY NIEORGANICZNE PowerPoint Presentation, free download – ID Nuevo

Veamos ランキング2022 少年隊 テレフォンカード テレカ 50度数 未使用品 ilr.ro volviéndose viral

Mira ランキング2022 少年隊 テレフォンカード テレカ 50度数 未使用品 ilr.ro tendencias

PPT – KWASY NIEORGANICZNE PowerPoint Presentation, free download – ID tendencias