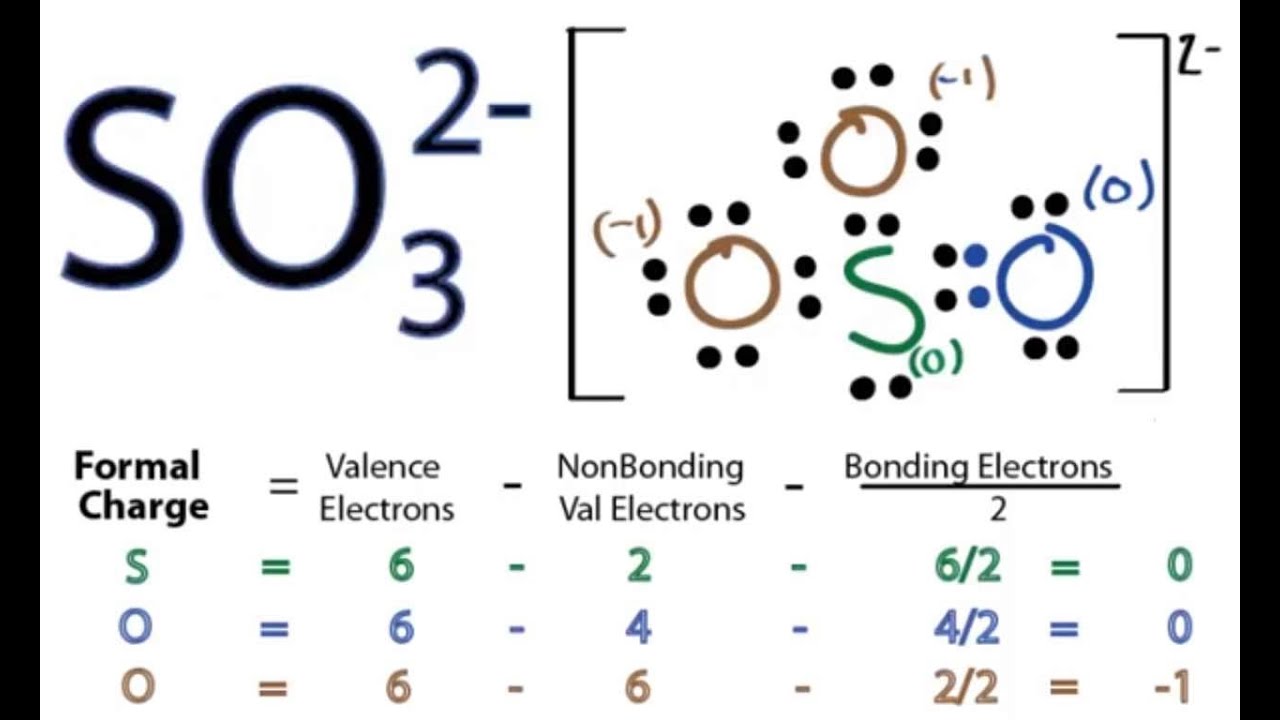
Put the least electronegative atom in the center. Webhet sulfaation is een polyatomisch oxoanion van zwavel met als brutoformule so en een molaire massa van 96,06 g / mol. Het ion bestaat uit een centraal zwavelatoom omgeven door vier zuurstofatomen in een tetraëdrische geometrie. Het sulfaation bezit een tweewaardig negatieve lading.
Frases La Bella Y La Bestia, 4.9 MB, Momento Epico Disney Transformacion De La Bestia La Bella Y La Bestia, 03:34, 446,425, Disnovemac LA, 2016-05-27T18:35:48.000000Z, 19, Pin en Frases de Películas Disney, www.pinterest.com.mx, 1000 x 1000, png, , 20, frases-la-bella-y-la-bestia, Zadania lekcyjne
Die salze enthalten als anion das sulfition (so 32− ). Sie werden häufig als konservierungsmittel in wein, trockenobst und kartoffelprodukten eingesetzt. Sulfite treten allerdings auch natürlich in nahezu allen weinen auf. Web2 so 2 + o 2 2 so 3. En sortie de réacteur, le trioxyde de soufre est absorbé par de l'acide sulfurique initialement à 98,5 %, et non par de l'eau car la réaction, so 3 + h 2 o h 2 so 4 bien que très exothermique (δh = −88 kj mol −1 ), est lente. La solution résultante de trioxyde de soufre dans l'acide sulfurique s'appelle oléum. (nh4)2 (so3) what is the chemical formula of stannic sulfite? Sn (so3)2 how do i insert the coefficients to balance the equation so2 plus o2 so3?. National institutes of health.
Mirar SO3 2- Lewis Structure – How to Draw the Lewis Structure for SO3 2- (Sulfite Ion)
Noticias SO3 2- Molecular Geometry / Shape and Bond Angles Nuevo
Aquí How to Draw Lewis Structure for sulfite so3(2-)
Obligatorio SO3 2- : Lewis Structure and Molecular Geometry Último
Ver Lewis Dot Structure of SO3 2- (Sulfite Ion) tendencias
Últimas QUIMICA Estructura de Lewis ion sulfito (SO3 -2) Carga formal Hibridación AULAEXPRESS
Acerca de How to Calculate the Formal Charges for SO3 2- (Sulfite ion) tendencias
Veamos How to find the Oxidation Number for S in the SO3 2- ion. (Sulfite ion)
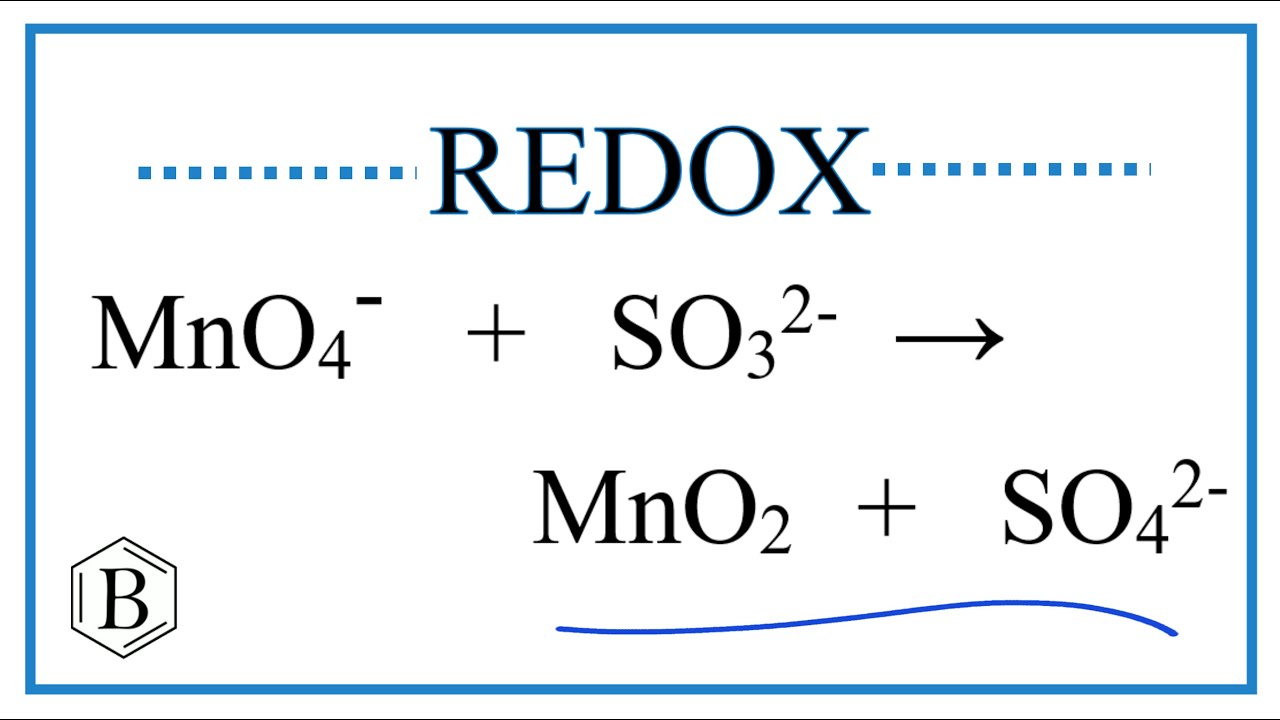
Artículos Balance the Redox Reaction for MnO4- + SO3 2- → MnO2 + SO4 2- viral
Discusión SO3 2- Lewis structure. Draw the structure of so3 2- . Oxidation Number for so3 -2 . Sulfite ion tendencias
Otras descripciones de So3 2- Siguiente
A step-by-step explanation of how to draw the SO3 2- Lewis Structure (Sulfite Ion).
For the SO3 2- Lewis structure the total number of valence electrons (found on the periodic table) for the SO3 2- molecule. In the Lewis structure of SO3 2- structure there are a total of 26 valence electrons. SO3 2- is also called Sulfite ion.
Once we know how many valence electrons there are in SO3 2- we can distribute them around the central atom with the goal of filling the outer shells of each atom.
For the Lewis structure for SO3 2- you should take formal charges into account to find the best Lewis structure for the molecule. It will be necessary to create a double bond on the Oxygen (O) atom for the formal charges to work.
Also note that you should put the SO3 2- Lewis structure in brackets with as 2- on the outside to show that it is an ion with a negative two charge.
—– Steps to Write Lewis Structure for compounds like SO3 2- —–
1. Find the total valence electrons for the SO3 2- molecule.
2. Put the least electronegative atom in the center. Note: Hydrogen (H) always goes outside.
3. Put two electrons between atoms to form a chemical bond.
4. Complete octets on outside atoms.
5. If central atom does not have an octet, move electrons from outer atoms to form double or triple bonds.
—– Lewis Resources —–
• Lewis Structures Made Simple: youtu.be/1ZlnzyHahvo
• More practice: youtu.be/DQclmBeIKTc
• Counting Valence Electrons: youtu.be/VBp7mKdcrDk
• Calculating Formal Charge: youtu.be/vOFAPlq4y_k
• Exceptions to the Octet Rule: youtu.be/Dkj-SMBLQzM
Lewis Structures are important to learn because they help us understand how atoms and electrons are arranged in a molecule, such as Sulfite ion. This can help us determine the molecular geometry, how the molecule might react with other molecules, and some of the physical properties of the molecule (like boiling point and surface tension).
Chemistry help at Breslyn.org
43+ So3 2- Molecular Geometry Background – GM viral

Aquí SO3 Lewis Structure, Molecular Geometry, and Hybridization Nuevo

Veamos Draw the Lewis dot structure for SO3 – Brainly.in tendencias

Aquí SO3 Lewis Structure – How to Draw the Lewis Structure for SO3 (Sulfur tendencias

Mira Estructura de Lewis SO3 » Quimica Online .NET más

Último 1) What is Resonance?2) why there is need of Resonance structures?3 actualizado

Acerca de SO3 Molecular Geometry / Shape and Bond Angles (Sulfur Trioxide) – YouTube volviéndose viral

Reseñas SO3 2- Lewis Structure – How to Draw the Lewis Structure for SO3 2

SO3 Hybridization: Hybrid Orbitals for SO3 (sulfur trioxide) – clipzui.com Último