
Hydrochloric acid, hcl, a strong acid, will react with ammonia, nh3, a weak base, to form aqueous ammonium chloride, nh4cl, according to the following chemical equation nh3(aq) + hcl(aq) → nh4cl(aq) Bài tập liên quan phản ứng hcl và nh3 trong các công thức hóa học mà bạn gặp phải trong bài tập chắc hẳn sẽ có phương trình khí amoniac ( nh3 ) hản ứng hóa học với hcl, cân bằng phưng trình hóa học nh3 + hcl như thế nào mời các bạn cùng theo dõi ở bài viết hôm nay. Mg + hcl nh3 + hcl viết phương trình phản ứng. Nh3 + hcl → nh4cl
Paradoja De La Piedra, 7.05 MB, La paradoja de la piedra y la omnipotencia de Dios, 05:08, 1,127, BIBLIA VS. RELIGIÓN, 2021-11-01T13:58:54.000000Z, 19, Mito Ateo: Paradoja de la Omnipotencia – YouTube, www.youtube.com, 1280 x 720, jpeg, paradoja, 20, paradoja-de-la-piedra, Zadania lekcyjne
Ammonia is a weak base that reacts with hydrochloric acid, forming a compound called ammonium chloride. Produced hydrogen chloride vapor can behave as an acidic compound (can release h + ions in the water). Then, hydrogen chloride reacts with basic ammonia gas to produce ammonium chloride which is a solid white smog. To happen this second step reaction, ammonia is required. That's why required amount of ammonia is high. 2nh 3 + 3cl 2 = n 2 + 6hcl De structuur van de verbinding is niet vlak, maar neemt als gevolg van het vrij elektronenpaar op stikstof een trigonaal piramidale moleculaire geometrie aan. Het molecuul is uitgesproken polair. Hcl + hno3 = no + cl2 + h2o | hydrogen chloride react with nitric acid 6hcl + 2hno 3 2no + 3cl 2 + 4h 2 o [ check the balance ] hydrogen chloride react with nitric acid to produce nitric oxide, chlorine and water.
Ammonium Chloride HCl + NH3 = NH4Cl Hydrochloric acid + ammonia hydroxide. popular
Reseñas HCl+NH3=NH4Cl – Ammonium chloride smoke under 100,000,000x microscope actualizado
Artículos Balance NH3 + HCl = NH4Cl (Ammonia and Hydrochloric Acid) viral
Acerca de Easy way to understand the NH3 with HCl Titration viral
Acerca de Ammonia and hydrogen chloride diffusion experiment Último
Actualmente – Reaction of NH3 (g) + HCl (g) Can two gases make a solid 🌪 más
Nuevo Diffusion of NH3 and HCl actualizar
Videos Find the pH: NH3 and HCl (Titration: Strong Acid/Weak Base) Último
How to Write the Net Ionic Equation for NH3 + HCl = NH4Cl volviéndose viral
Últimas Superfast: NH3 + HCl Titration (Strong Acid-Weak Base)
Qué saber sobre Hcl Nh3 que podría ser interesante
Join the science discord! discord.gg/pw5sZ3PTye Easy DIY chemistry project, great for science fairs because its a great demonstration! This is great for the chemistry beginner.
I used 150ml of a 10% ammonia solution
and 50ml of 30% HCl
After they reacted, i boiled it down, and dried it in the oven. It tastes very salty/tart!
Imprescindible Balance NH3 + HCl = NH4Cl (Ammonia and Hydrochloric Acid) – YouTube Último

Acerca de How to Balance HCl + NH3 = NH4Cl (Hydrochloric acid + Ammonia) – YouTube popular

Is Nh4cl An Acid Or Base – slidesharetrick actualizar

Ver How to Write the Net Ionic Equation for NH3 + HCl = NH4Cl – YouTube Último

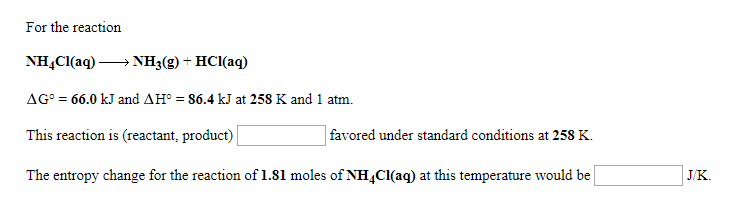
Ver Solved: For The Reaction NH4Cl(aq)>NH3(g) + HCl(aq) Δ Go 6… | Chegg.com viral

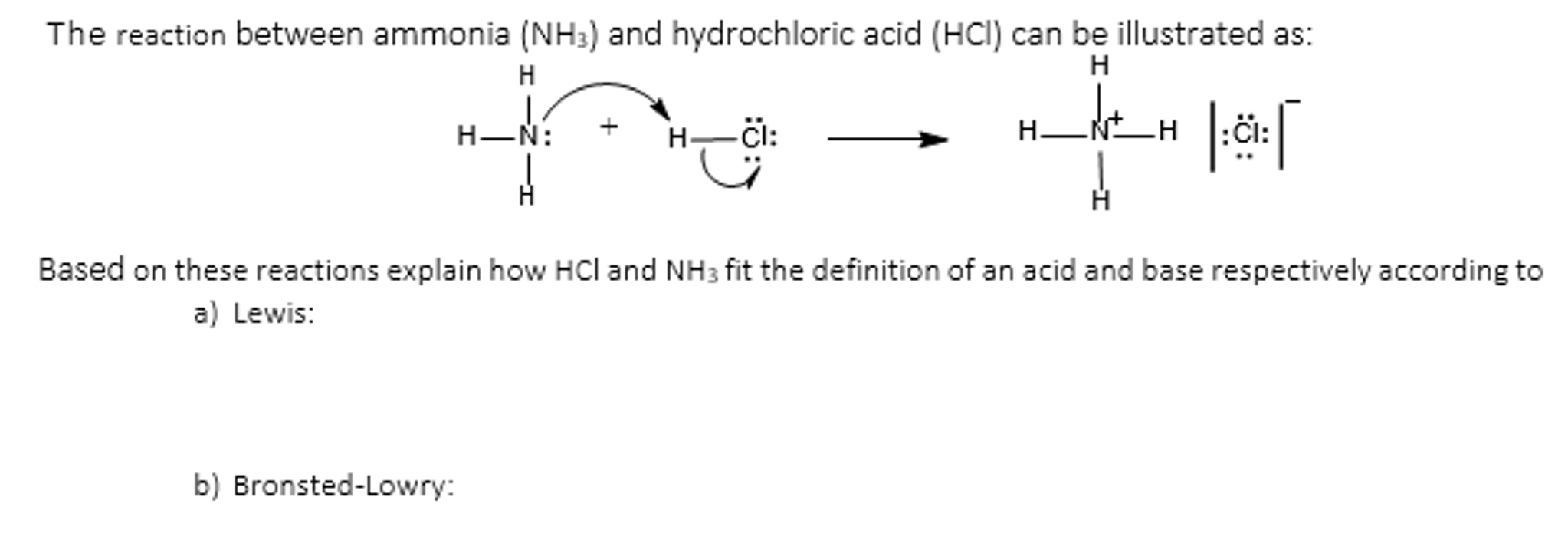
Ver Solved: The Reaction Between Ammonia (NH_3) And Hydrochlor… | Chegg.com
Reseñas 3rd attempt W See Periodic Table Ammonia, NH3, rapidly reacts with

Solved: For The Reaction NH4Cl(aq)>NH3(g) + HCl(aq) Δ Go 6… | Chegg.com tendencias
Nuevo Solved: Consider The Reaction HCl(g)NH3(g)>NH4C1(s) Using | Chegg.com volviéndose viral

Veamos Consider the reaction: NH3 (g) + HCl (g) → NH4Cl (s) Given | Clutch










