
Webcaco3 cao + co2 2. Điều kiện phản ứng caco3 ra cao nhiệt độ cao 3. Bài tập vận dụng liên quan phản ứng nhiệt phân câu 1. Nhiệt phân hoàn toàn m gam hỗn hợp x gồm.
Realidad Estatica Y Dinamica, 1.83 MB, Estática social y dinámica social en 1 minuto – Sociología en 1 minuto, 01:20, 1,037, Vía sociológica, 2022-03-12T02:40:39.000000Z, 19, Unidad 3 estática y dinámica parte 2 – YouTube, www.youtube.com, 1280 x 720, jpeg, , 20, realidad-estatica-y-dinamica, Zadania lekcyjne
Canxi oxit hấp thụ khí cacbon đioxit, tạo thành canxi cacbonat. Webin order to balance caco3 = cao + co2 you'll need to watch out for two things. First, be sure to count all of ca, o, and c atoms on each side of the chemical equation. Once you know how many of. Webcalciumoxide is een basevormend oxide met als brutoformule cao. Een andere naam voor calciumoxide is ongebluste kalk of gebrande kalk. Calciumoxide is een wit poeder. Caco 3 + co 2 +h 2 o—> ca (hco 3) 2. Webput a small piece of calcium oxide in air at ambient temperature in a full sentence, you can also say cao () reacts with co2 (carbon dioxide) and produce.
Veamos How to Balance CaCO3 = CaO + CO2 tendencias
Ver How to Balance CaCO3 = CaO + CO2 (Decomposition of Calcium Carbonate with 🔥 heat)
Ver CaCo3 =CaO +Co2.#youtube #experiment #aotvchannel
Type of Reaction for CaCO3 = CaO + CO2 volviéndose viral
Nuevo Easy tips to balance CaCO3 = CaO + CO2 viral
Videos Decomposition of calcium carbonate Nuevo
HEATING OF CALCIUM CARBONATE TO GIVE CALCIUM OXIDE & CARBON DIOXIDE|DECOMPOSITION REACTION|EDUPETAL tendencias
Acerca de Thermal decomposition of calcium carbonate (CaCO3) -fail
Temas Reacting Mass: Series 4 – CaCO3(s) — CaO(s) + CO2(g). Último
Videos How to Balance CaCO3=CaO+CO2|Chemical equation CaCO3=CaO+CO2|CaCO3=CaO+CO2 balance equation más
Explicación de Caco3 Cao Co2
In order to balance CaCO3 = CaO + CO2 you’ll need to watch out for two things.
First, be sure to count all of Ca, O, and C atoms on each side of the chemical equation. Once you know how many of each type of atom you have you can only change the coefficients (the numbers in front of atoms or compounds) in order to balance the equation.
Be careful when counting the Oxygen atoms on the product side of the equation. Don’t forget the CaO3 in CO2 on the product side of the chemical equation!
Drawing/writing done in Adobe Illustrator 6.0. Screen capture done with Camtasia Studio 4.0. Done on a Microsoft Surface Pro 3.
Noticias How to Balance Ca(OH)2 + CO2 = CaCO3 + H2O (Limewater plus Carbon Último

Acerca de write Kc,Kp units of reaction caco3=cao+co2(s) (s) (g) please solve actualizado

Aquí "x" değerini bulunuz.a) CaCo3 —–> Cao + Co2 40 gram x 17,6 gram actualizado

Discusión Write expression for equilibrum constant for1)caco3——cao+co22 más

Ver Type of Reaction for CaCO3 = CaO + CO2 – YouTube Último

Caco3 =cao+co2 at 977°c∆H=174 kj /mol then ∆E is – Brainly.in viral

Noticias Solved: 3. Given These Reactions With Their Respective Ent… | Chegg.com viral
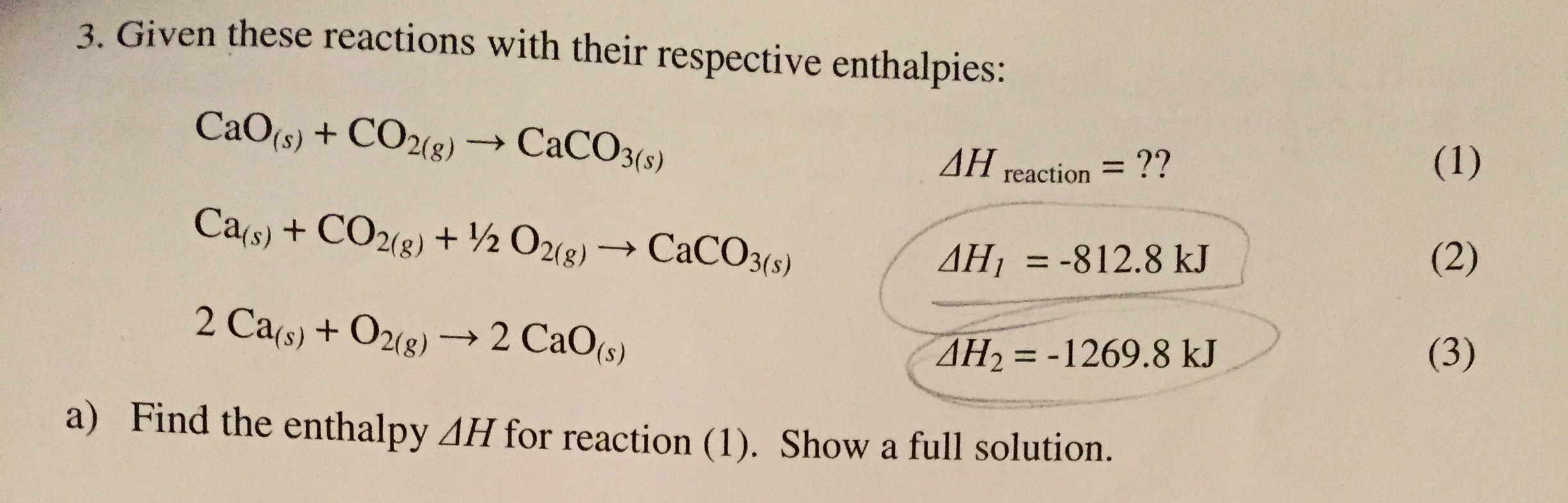
Actualmente – Write expression for equilibrum constant for1)caco3——cao+co22 Último

Reseñas Q7 Complete the following equations and balance them-i) ZnO (s popular

Viral CaCO3 = CaO + CO2From the above equation calculate the amount of carbon










