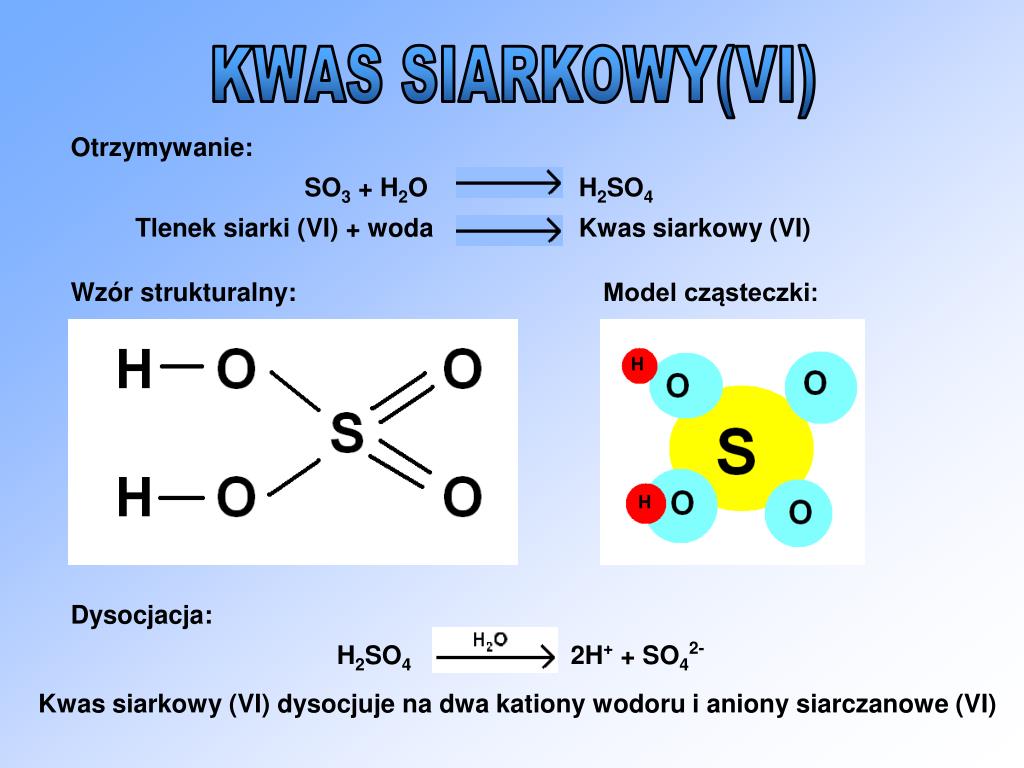
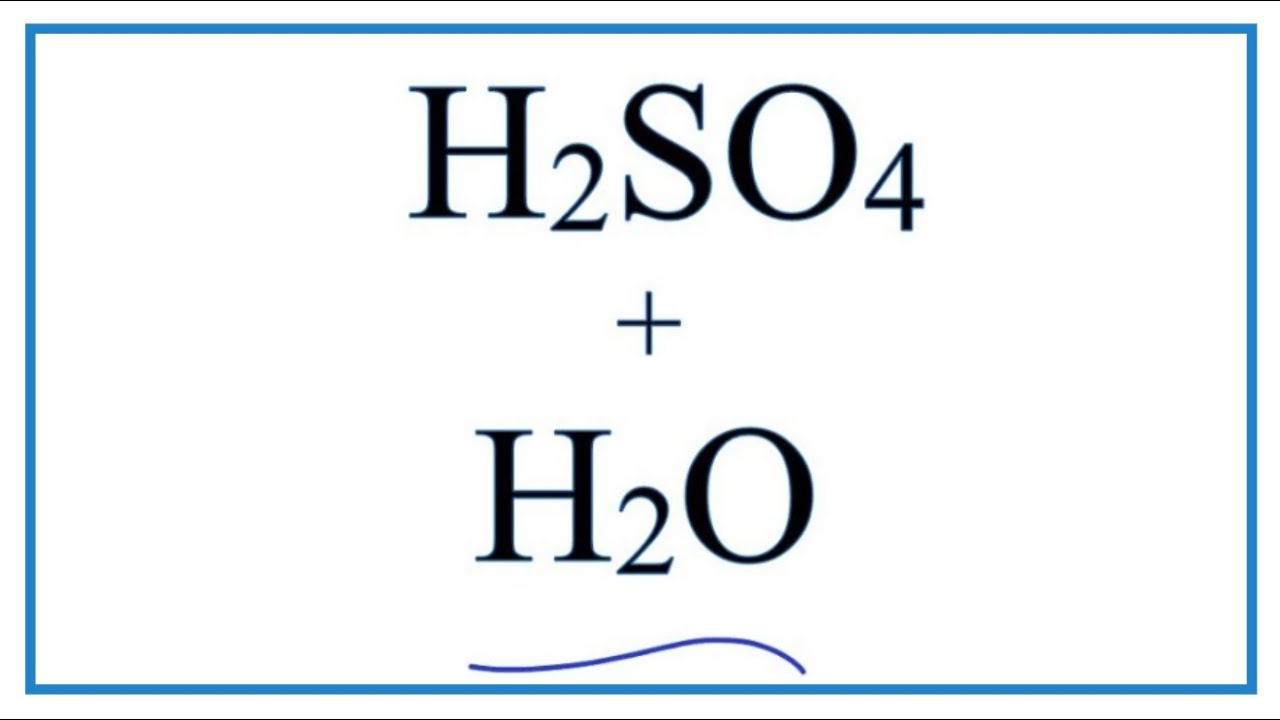
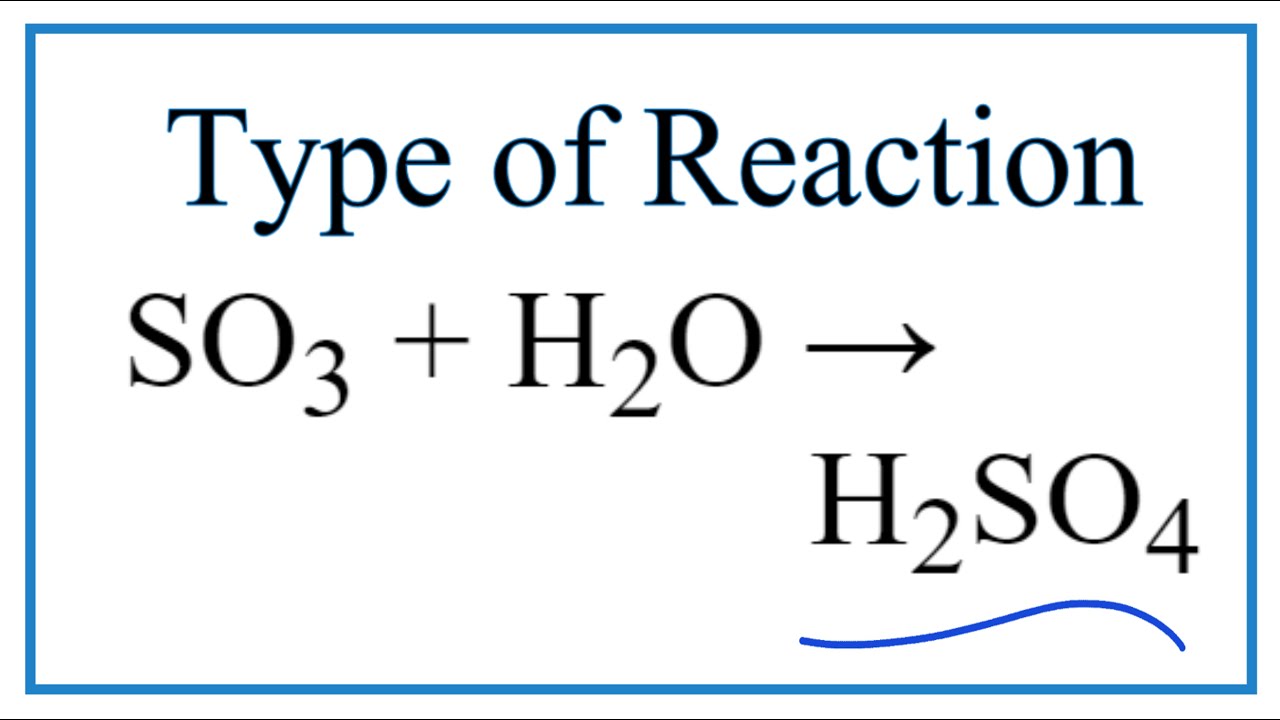Cũng như quá trình điều chế h 2 so 4 trong công nghiệp. Mời các bạn tham khảo. Phương trình so3 ra h2so4 so3 + h2o → h2so4 2. Điều kiện phản ứng so3 ra h2so4 nhiệt độ thường 3.
50 Psi A Bar, 5.24 MB, BAR, PSI, Pressure Measurement EXPLAINED, 03:49, 31,261, SpeedLab Channel, 2020-07-27T05:43:34.000000Z, 19, Pressure Gauge 50mm Dial 0/30 PSI & 0/2 Bar 1/4 BSPT BOTTOM and/or Hose, www.ebay.co.uk, 805 x 1000, jpeg, pressure gauge psi bar 50mm bspt dial bottom tails hose, 20, 50-psi-a-bar, Zadania lekcyjne
→ h2so4 óxido nometálico. Ácido s= 1 1 o= 4 4 h= 2 2 ya está balanceada publicidad ¿todavía tienes preguntas? Encuentra más respuestas ayuda es para hoy y es urgente, gracias: Calcula la cantidad de nitrato de plata que se necesita para preparar 1 l de disolución que contenga 2 g/10. So3 + h2o → h2so4 the reaction is highly exothermic in nature. Therefore, in the production of sulfuric acid, sulfur trioxide is usually absorbed in concentrated sulfuric acid to form oleum (h2s2o7), which is then diluted with water to get the acid (h2so4) having the desired concentration. Sulfur trioxide is the anhydride of sulfuric acid. In order to balance so3 + h2o = h2so4 you'll need to watch out for two things. First, be sure to count all of s, o, and h atoms on each side of the chem.
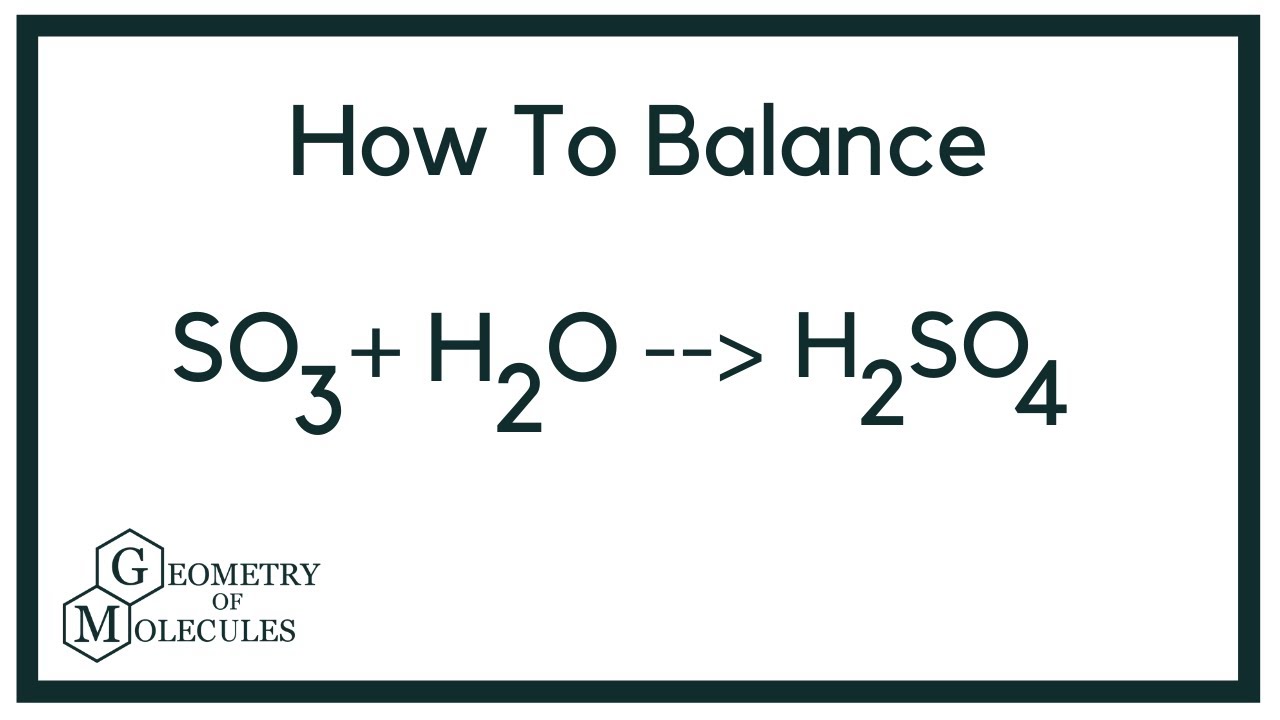
Obligatorio How to Balance SO3 + H2O = H2SO4 tendencias
Ver Type of Reaction for SO3 + H2O = H2SO4 popular
Artículos SO3 +H2O =H2SO4 Balanced Equation|| Sulfur trioxide ,Water, Sulfuric acid Balanced Equation más
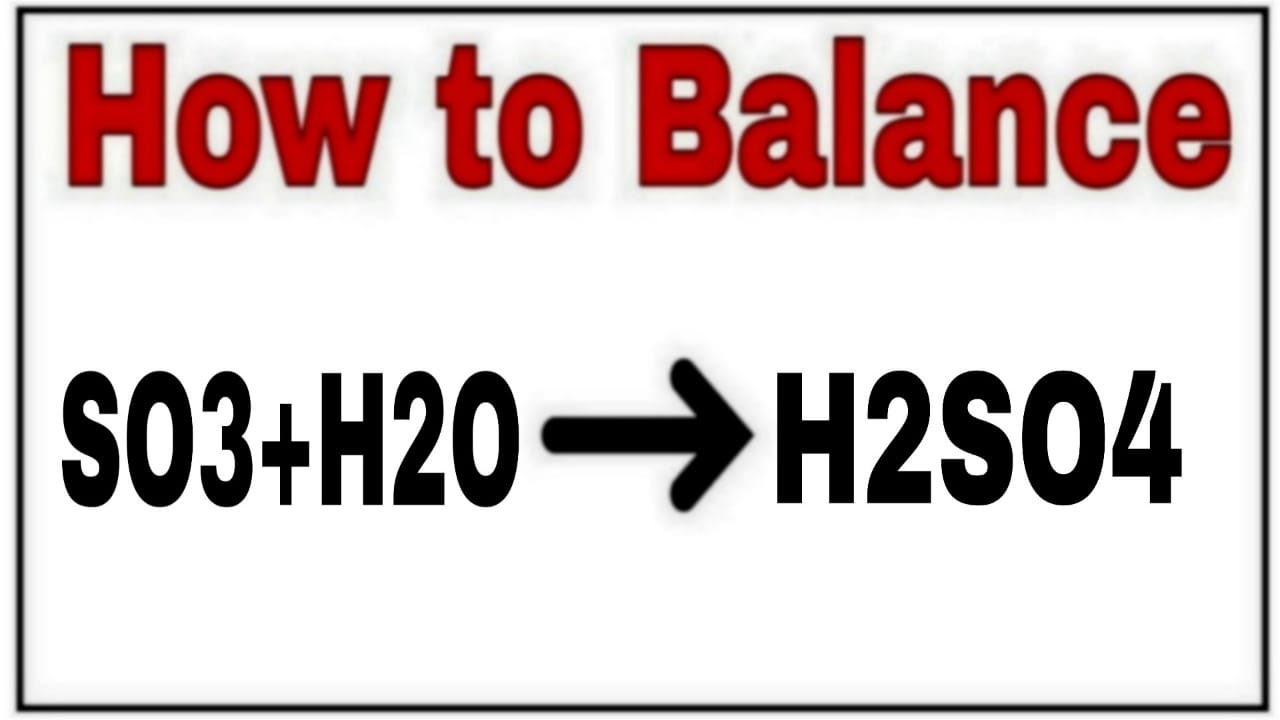
Discusión How to Balance SO3 + H2O = H2SO4 popular
Aquí How to balance SO3+H2O=H2SO4|Chemical equation SO3+H2O=H2SO4|SO3+H2O=H2SO4 Balanced|SO3+H2O=H2SO4 popular
How to Balance SO3+H2O=H2SO4|chemical equation SO3+H2O=H2SO4| SO3+H2O=H2SO4 Balance equation popular
Noticias Oleum. Sulfur trioxide SO3. Chemical reactions
Nuevo SO3 + H2O = H2SO4
Videos Reacciones de Combinación, Adición o Síntesis (Parte 1) Último
H2SO4 + H2O (Sulfuric acid plus Water)
Más sobre So3 H2o H2so4 del video anterior
In order to balance SO3 + H2O = H2SO4 you’ll need to watch out for two things.
First, be sure to count all of S, O, and H atoms on each side of the chemical equation. Once you know how many of each type of atom you have you can only change the coefficients (the numbers in front of atoms or compounds) in order to balance the equation.
Be careful when counting the Oxygen atoms on the product side of the equation. Don’t forget the H2O in SO3 on the reactant side of the chemical equation!
Drawing/writing done in Adobe Illustrator 6.0. Screen capture done with Camtasia Studio 4.0. Done on a Microsoft Surface Pro 3.
Acerca de So3 ƒgƒ ƒtƒB [ 342182

Viral CP1: Volumetric Flow of SO3(gas) to H2SO4 – YouTube Último

Nuevo How to balance SO3+H2O=H2SO4|Chemical equation SO3+H2O=H2SO4|SO3+H2O popular

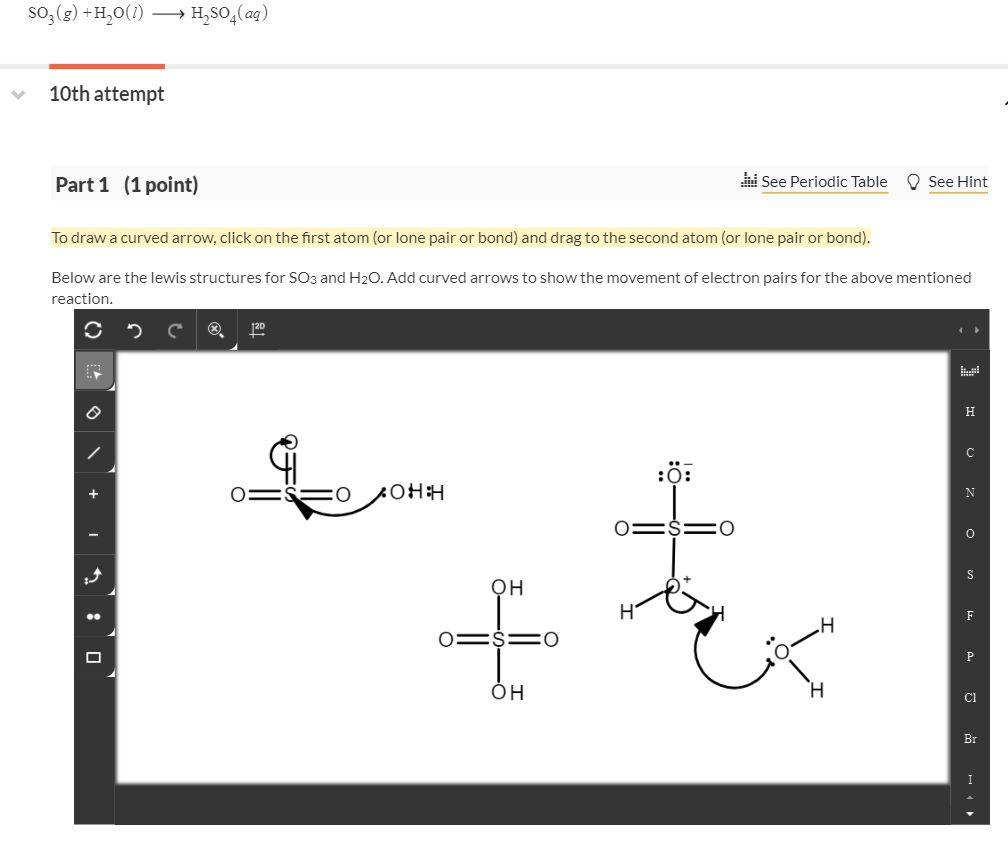
Viral Draw Lewis Structure Of H2so4 tendencias
Fotos ユニーク H2so4 – マシアフテナン viral
Acerca de óXidos actualizado

Imágenes SO3 H2O = H2SO4 | SO3+H2O=H2SO4 más

Discusión Классифицируйте реакции по тепловому эффекту. В таблицу внесите ответы tendencias

Artículos Solved: O 1. SO3, H2SO4 C 2, Cl2, FeCl3 3. H2SO4, H2O, Hea… | Chegg.com Último

Viral PPT – KWASY TLENOWE PowerPoint Presentation, free download – ID:1349269 actualizado