Nh3 is the chemical formula of ammonia and h2o is the chemical formula of water. Nh3 + h2o is the hydrolysis reaction within ammonia and water. Ammonia is the naturally occurring compound or it can make artificially in laboratories. It is a transparent gas with no colour and have strong pungent odour.
Derivado Del Petroleo Que No Es Biodegradable, 8.88 MB, Derivados del petróleo, 06:28, 5,210, Geografía Virtual, 2020-09-22T20:42:12.000000Z, 19, Derivado del petróleo que no es biodegradable | Los mejores materiales, www.todobiodegradable.org, 746 x 1200, jpeg, , 20, derivado-del-petroleo-que-no-es-biodegradable, Zadania lekcyjne
Natriumhydroxide, ook wel caustische of bijtende soda genoemd, is een anorganische verbinding met als brutoformule naoh. De stof komt voor als een witte, hygroscopische vaste stof, die zeer goed oplosbaar is in water. Tijdens het oplossen komt een grote hoeveelheid warmte vrij. De uiteindelijke oplossing wordt ook wel aangeduid als natronloog. Key properties are provided in table 1. Hydrogen can be released on demand from ammonia through catalytic decomposition and consumed in a proton exchange membrane (pem) fuel cell 3. Thu aug 04, 2011 8:53 pm has upvoted: 反应的kb = 1. 8×10。. Nh3(アンモニア)+h2o(水)=nh4oh(アンモニア水) これについての質問なのですが… h4の部分ってh2oのhからnh3のhに1つ持ってきたということなのでしょうか。 だとしたら、何故nh5oと言うように全てまとめてはいけないのでしょうか。 簡単に、説明お願いします
Viral Equation for NH3 + H2O (Ammonia + Water)
Viral How to Balance NH3 + H2O = NH4OH (ammonia and water)
Ver NH3 + H2O Ammonia and water המסת אמוניה במים
Mirar Ammonia Fountain in RamZland!⚗️NH3 + H2O ⇌ NH4+ + OH– tendencias
Temas NH3-H2O simple and actual vapour absorption system actualizar
Asunto Bond angle of NH3, H2O and CH4 tendencias
Ver Why are | Bond angles of h20 and nh3 104.5 and 107.5 | Angular shape of h20 | Pyrimidal shape of nh3 más
Noticias Non Aqueous Solvents Part – VII #Comparison of NH3 & H2O volviéndose viral
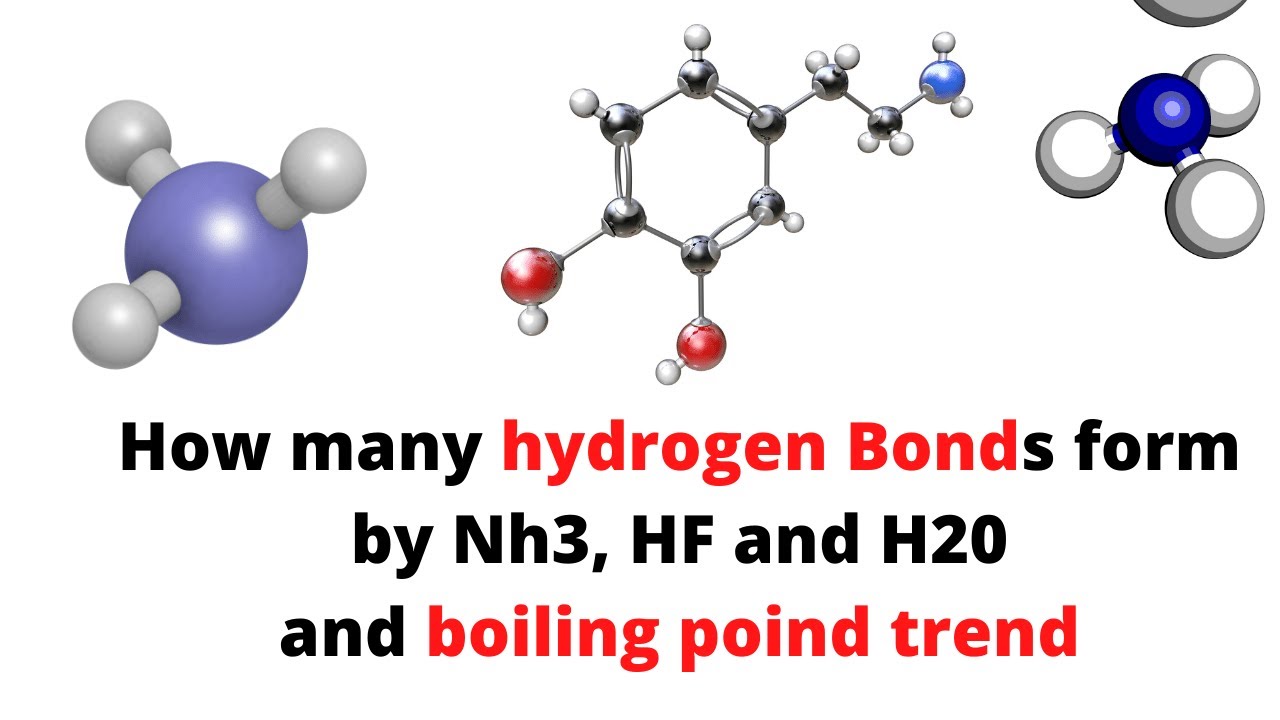
Discusión How many hydrogen bonds form by Nh3, H20 and HF and boiling point trend actualizado
Aquí How to Balance NH3 + CO2 + H2O = NH4HCO3 | Ammonia + Carbon dioxide + Water
Detalles de Nh3 H2o del video anterior
In this video we will describe the equation NH3 + H2O and write what happens when NH3 is dissolved in water.
When NH3 is dissolved in H2O (water) a small amount will dissociate into NH4+ and OH- ions. To show that they are dissolved in water we can write (aq) after each. The (aq) shows that they are aqueous – dissolved in water.
Even though NH3 (Ammonia) very soluble in water, it is a weak base. Only 0.42% of the NH3 molecules will form NH4OH. This an equilibrium. At any given time 0.42% of the NH3 molecules will be in the form of NH4OH. (see en.wikipedia.org/wiki/Ammonia_solution.
Often we consider equations showing ions to be net ionic equations. For help with net ionic equations see youtu.be/PXRH_IrN11Y .
If you need to know how to balance chemical reactions, see my complete tutorial on balancing all types of chemical equations:
Balancing Equations in 5 Easy Steps: youtu.be/zmdxMlb88Fs
More Practice Balancing: youtu.be/Qci7hiBy7EQ
Drawing/writing done in InkScape ( inkscape.org/). Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
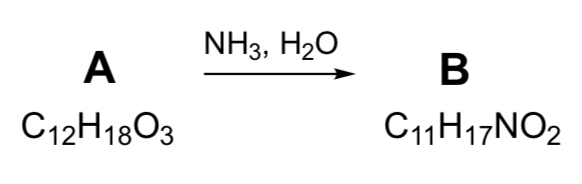
Imprescindible Answered: NH3, H2O C12H1803 C11H17NO2 | bartleby tendencias

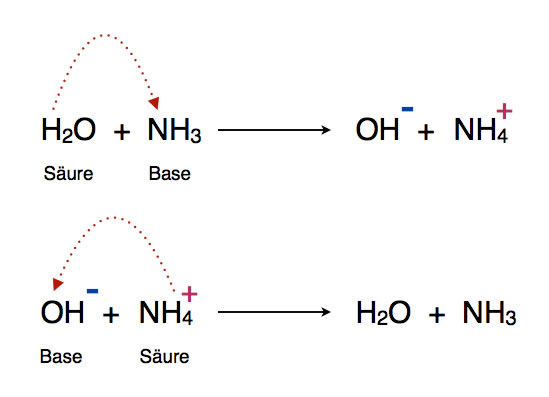
Nuevo Welche Bedeutung hat die chemische Formel,,H2O+NH3- – – > OH- +NH4“ für tendencias
Acerca de chemmacros reaction environment align – TeX – LaTeX Stack Exchange tendencias

Ver Question 4.8 Although geometries of NH3 and H2O molecules are distorted

Ver organic chemistry – Competition of H2O and NH3 leaving groups in

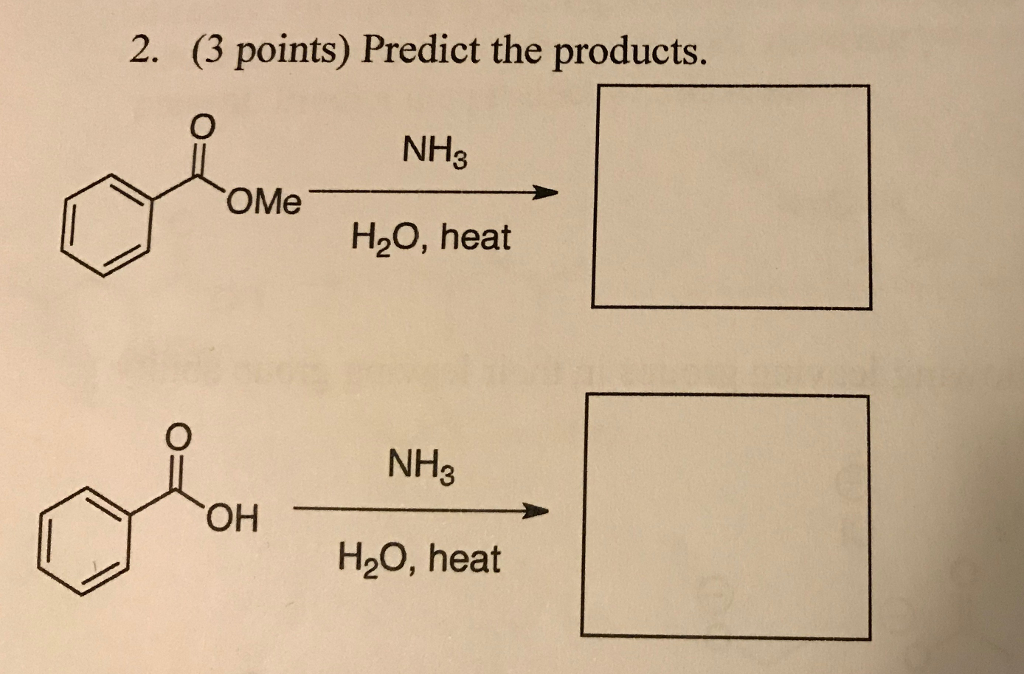
Asunto Solved: (3 Points) Predict The Products. NH3 H2O, Heat 2. | Chegg.com tendencias
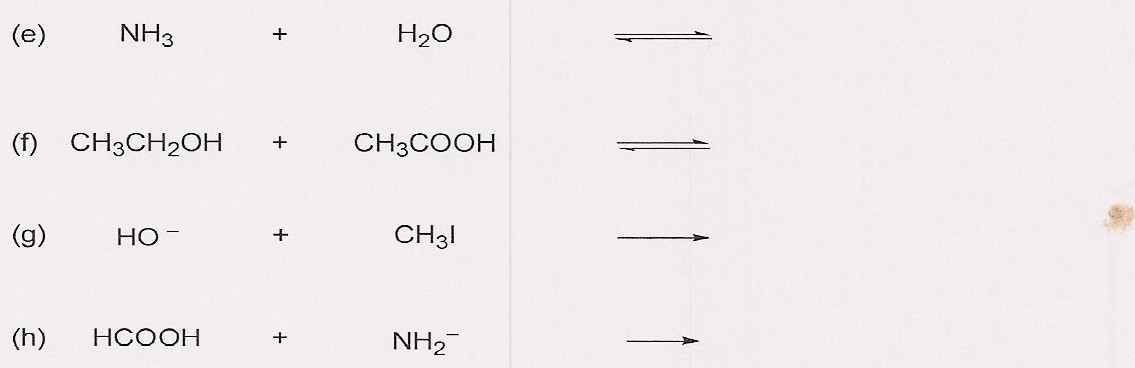
Imágenes Solved: NH3 + H2O CH3 CH2 OH + CH3 COOH HO- + CH3l HCOOH +… | Chegg.com Nuevo
balance the redox reaction KMNO4 + NH3 —> KNO3 + MNO2 + KOH + H2O

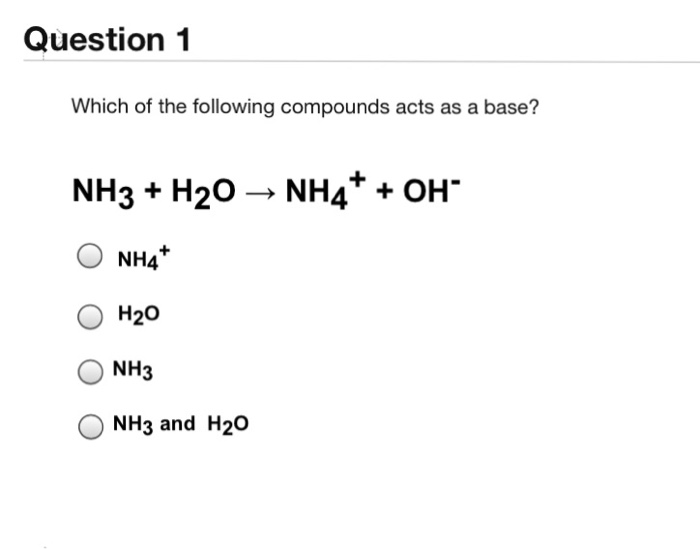
Viral Solved: Question 1 Which Of The Following Compounds Acts A… | Chegg.com volviéndose viral
Discusión Now examine the other reaction, NH3(aq) + H2O(ℓ) ⇌ NH4^+(aq volviéndose viral