Al (hco3)3 what is the formula for aluminum bicarbonate? I greatly doubt that aluminium bicarbonate exists. El bicarbonato es la base conjugada del ácido carbónico. The mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass:
Secretos Ocultos Crucigrama, 19.98 MB, RESUELTO TODO el EVENTO OCULTO! TODAS LAS PALABRAS Y COMBINACIONES | COD Mobile | EVENTO SECRETO, 14:33, 50,311, Rido, 2022-07-21T13:00:24.000000Z, 19, Mensaje secreto números enteros, es.slideshare.net, 638 x 903, jpeg, , 20, secretos-ocultos-crucigrama, Zadania lekcyjne
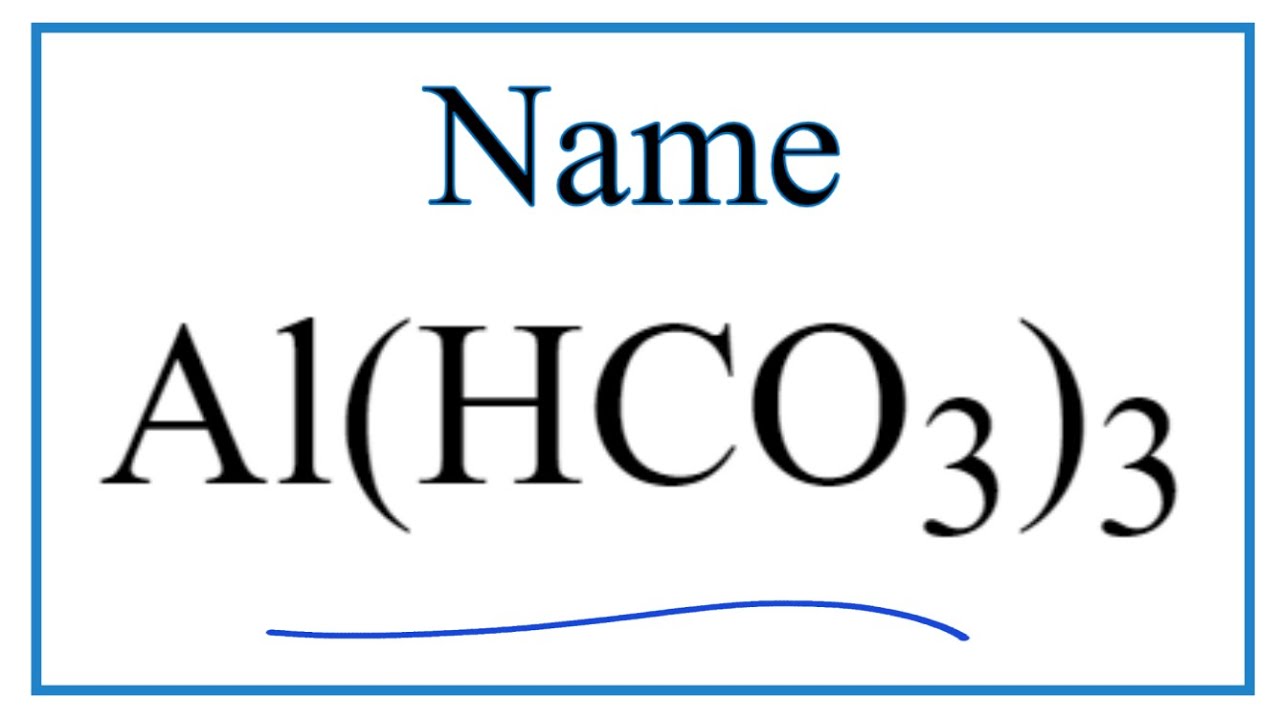
Molar mass of nacl is 58. 443, how many grams is 5 mole nacl? Grams = 58. 443 × 5 = 292. 215 (g) molar mass of agno3 is 169. 873, 2 kg agno3 is equal to how many moles? Use the exact name for the metal from the periodic table. Look up the name for the ion on the common ion table. Put them together (metal first) and you've got the name for al (hco 3) 3. Show answer al (hco3)3 resources how to name ternary ionic compounds El nombre químico del al (hco₃)₃ es: Carbonato ácido de aluminio. ¿qué es la nomenclatura química?
Videos How to Write the Name for Al(HCO3)3
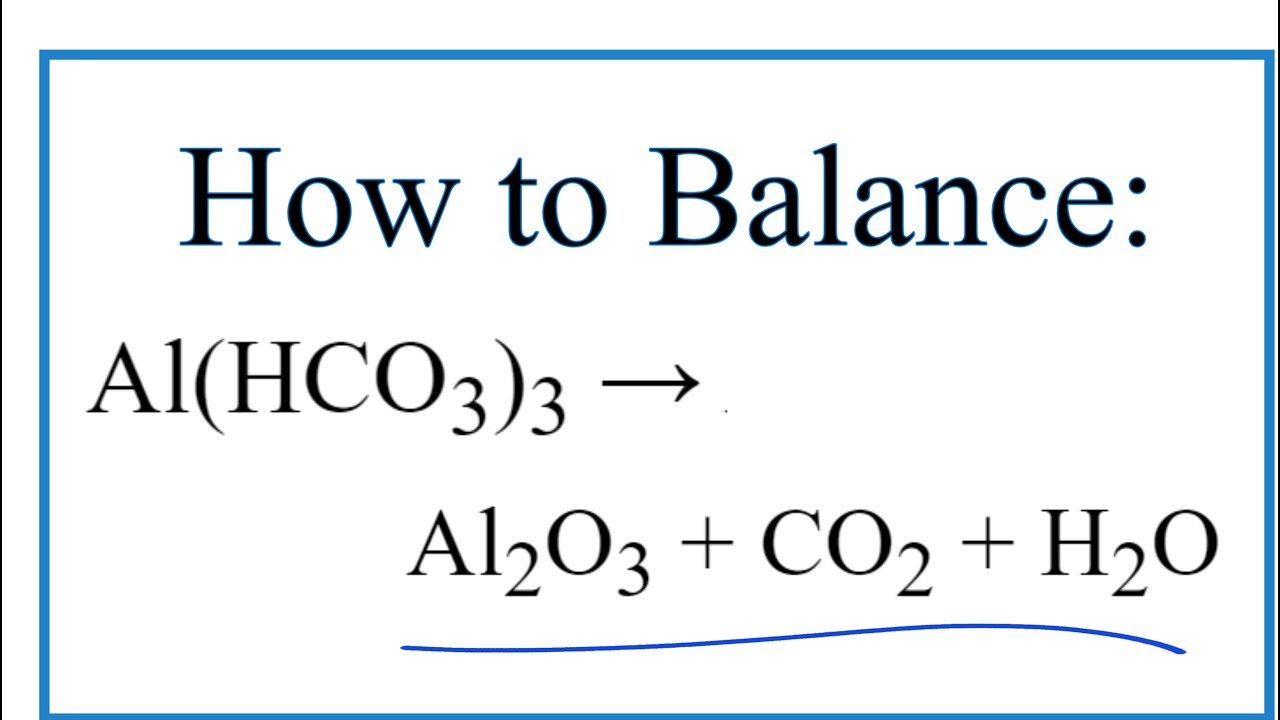
Viral How to Balance Al(HCO3)3 = Al2O3 + CO2 + H2O más
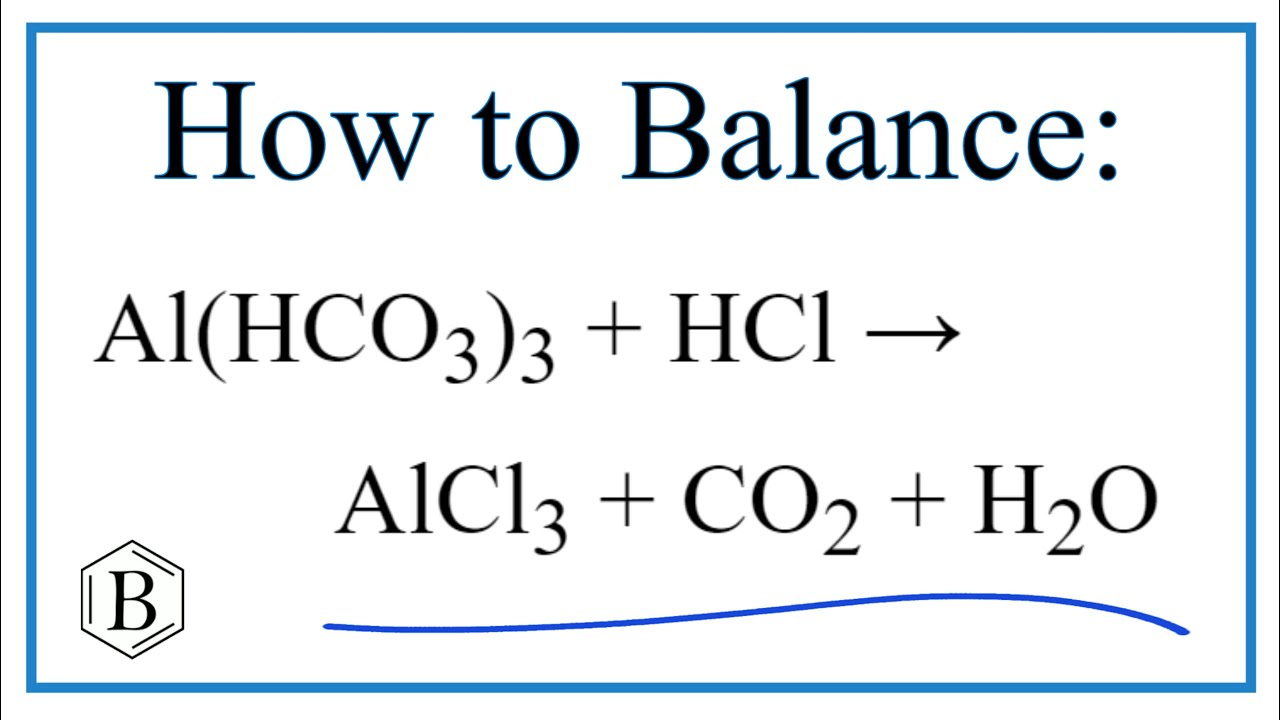
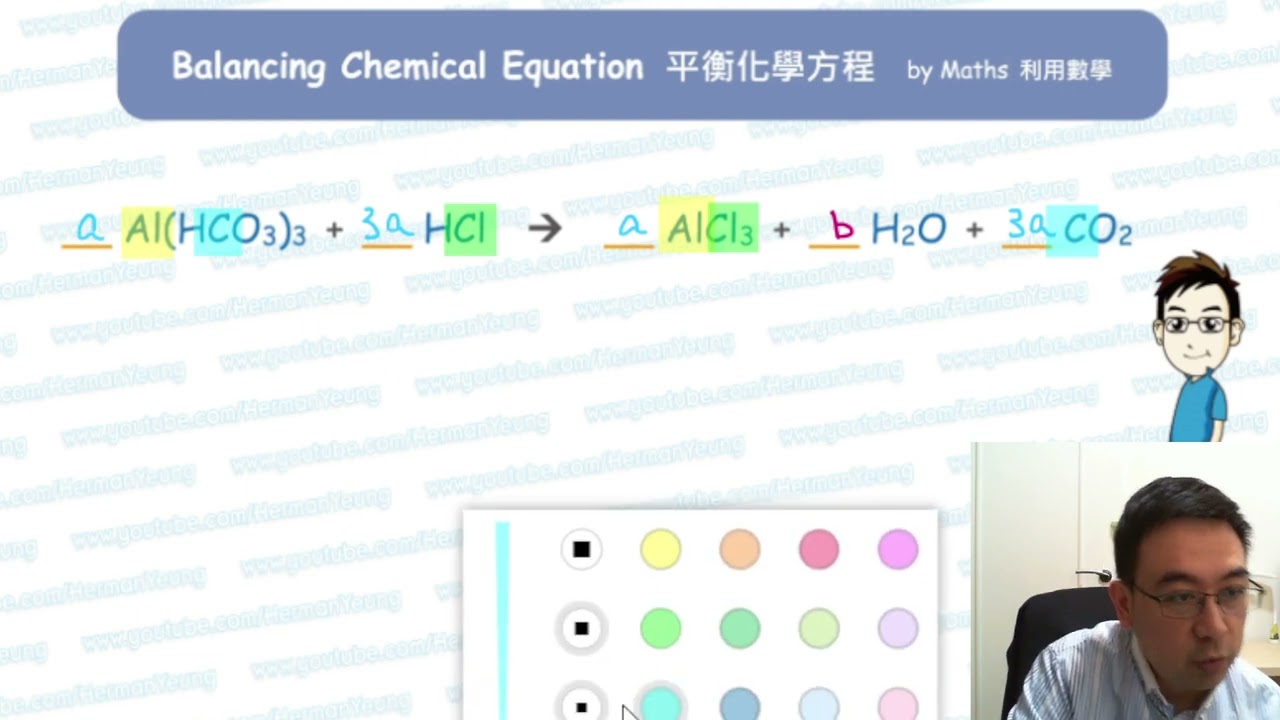
Videos Balancing the Equation Al(HCO3)3 + HCl = AlCl3 + CO2 + H2O (and Type of Reaction) actualizar
How to balance Al(HCO3)3 + HCl = AlCl3 + H2O + CO2 Último
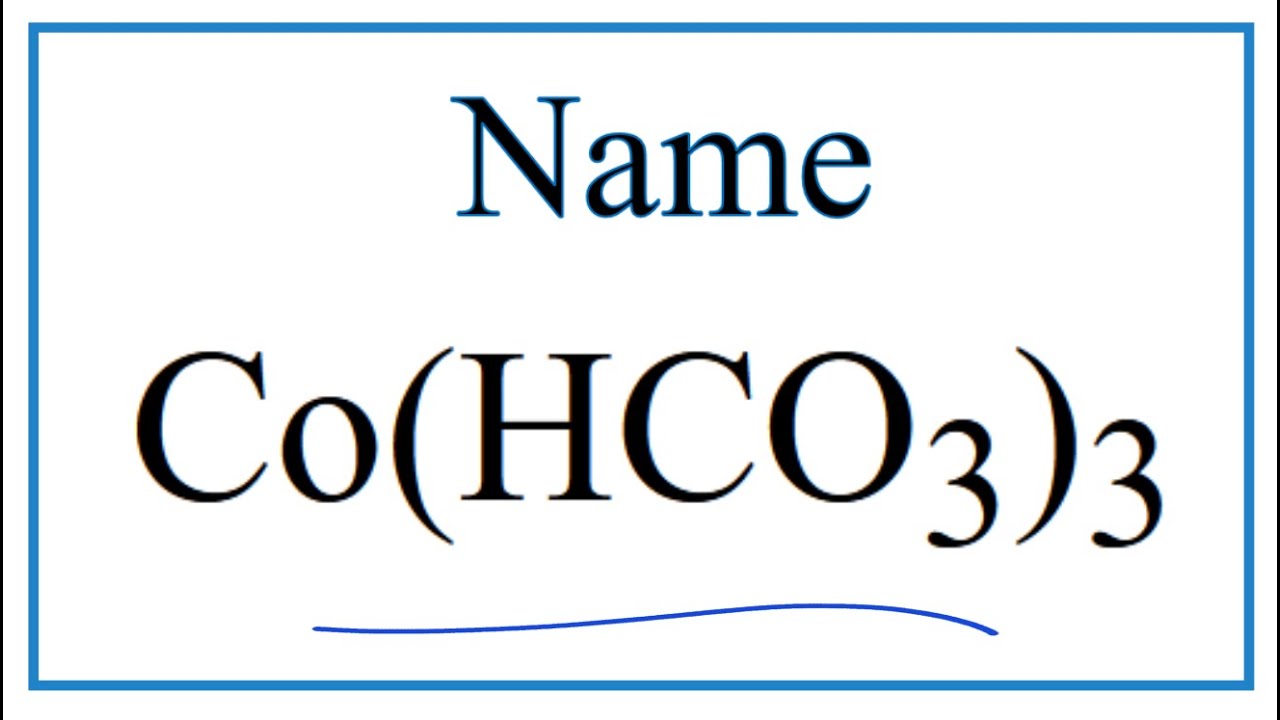
Noticias How to Write the Name for Co(HCO3)3 más
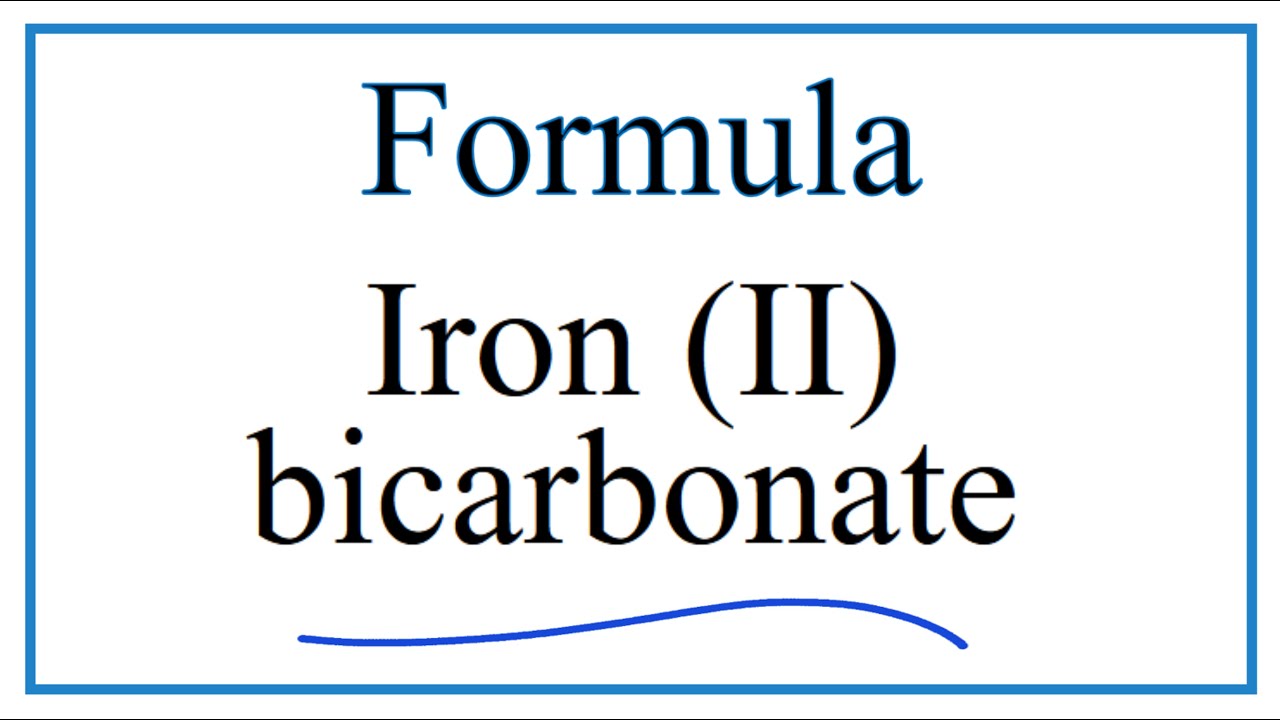
Acerca de How to Write the Name for Fe(HCO3)3 actualizar
Discusión How to name Mg(HCO3)3 te
ndencias
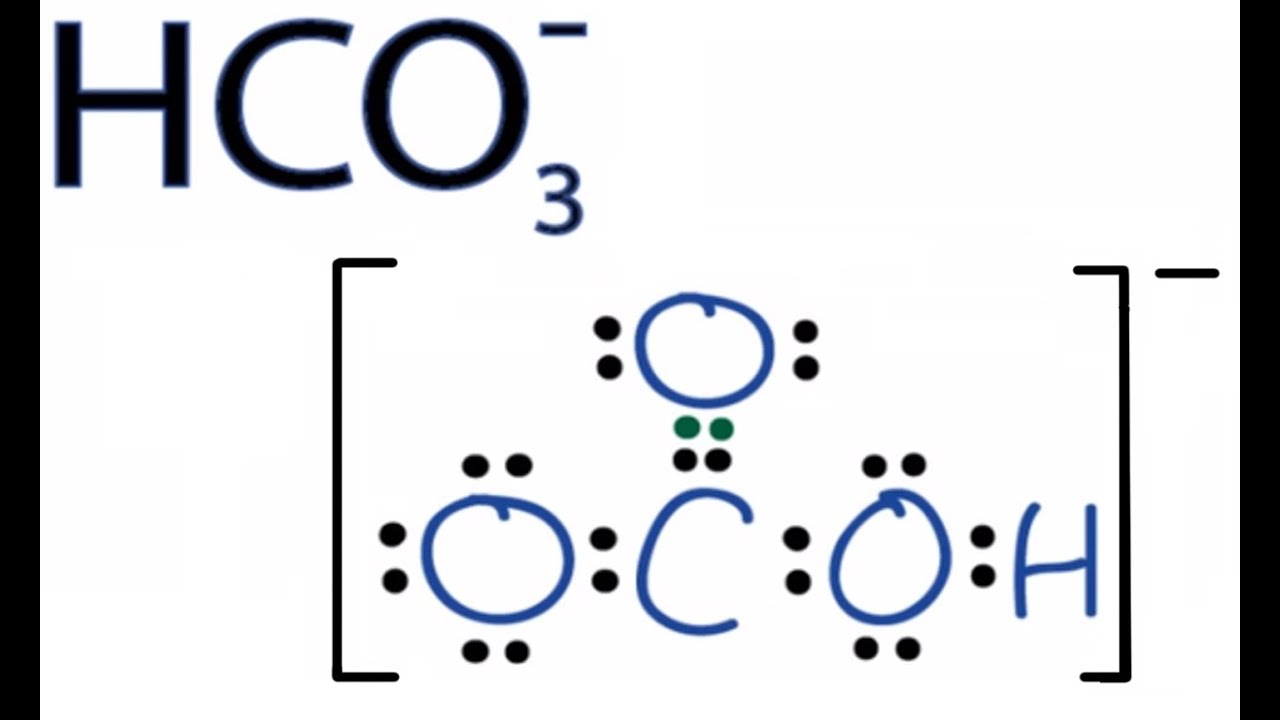
HCO3- Lewis Structure: How to Draw the Lewis Structure for HCO3- popular
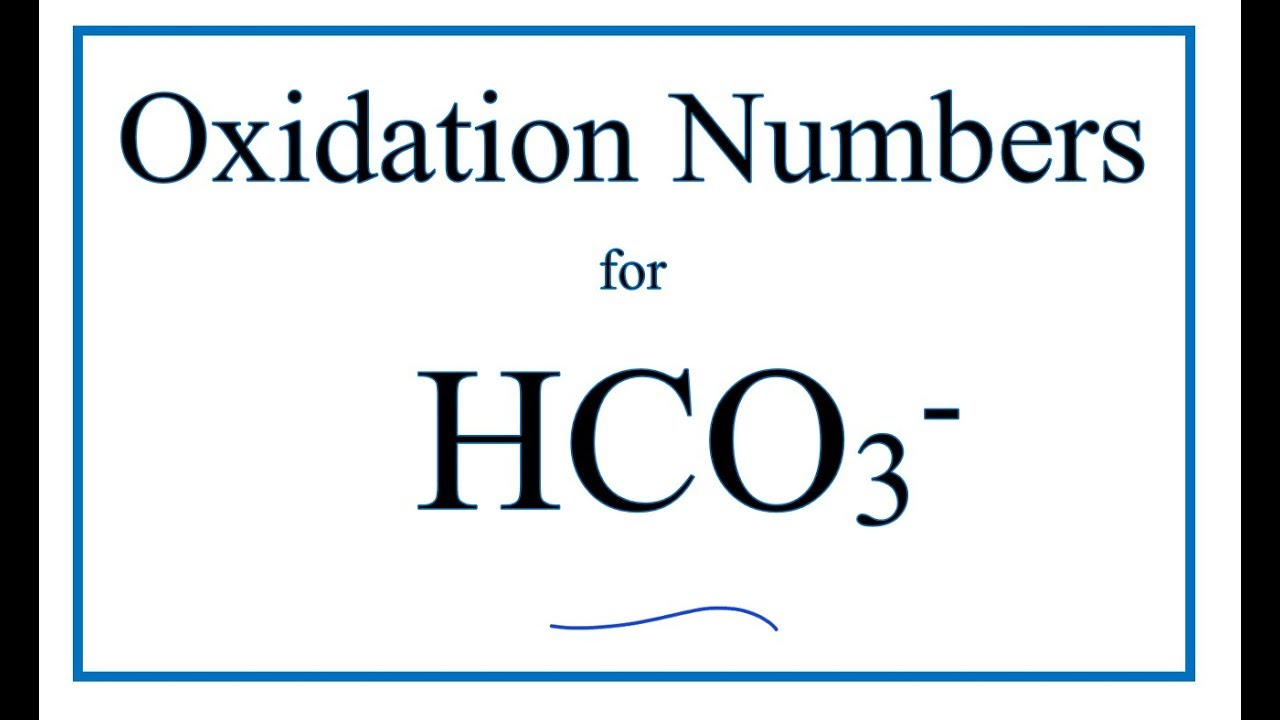
Ver How to find the Oxidation Number for C in the HCO3- ion. (Bicarbonate ion) viral
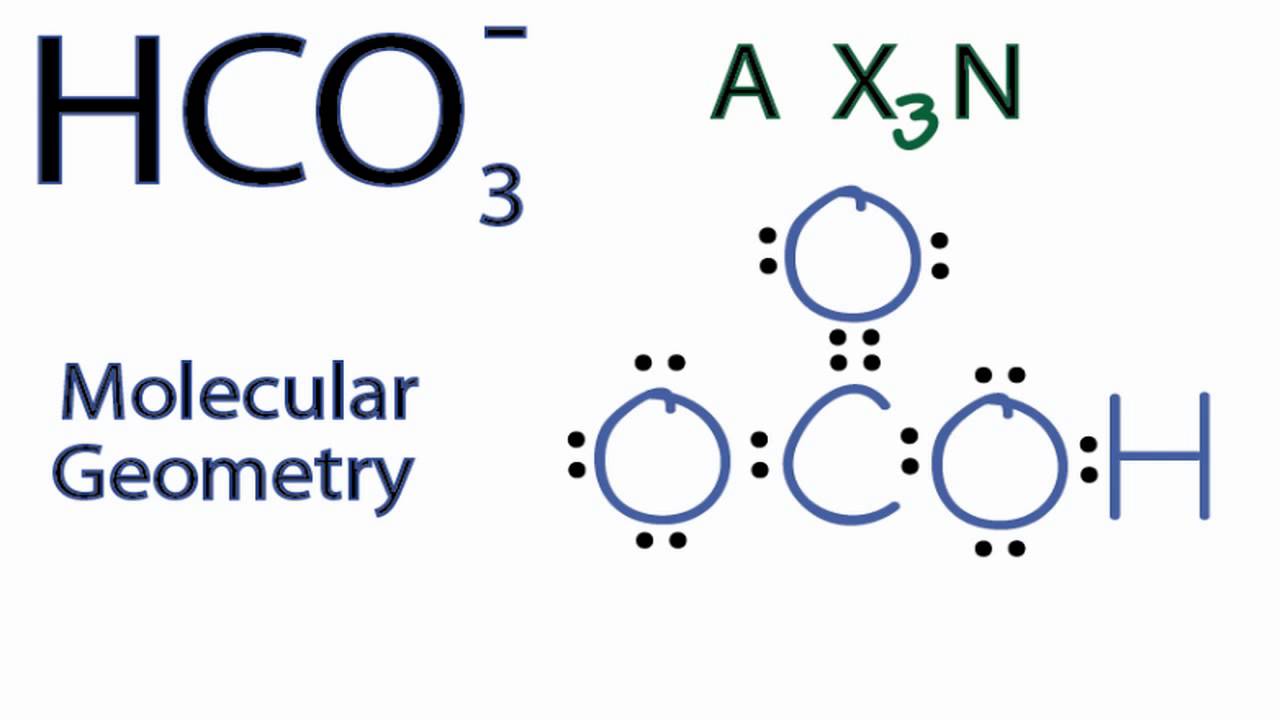
Acerca de HCO3- Molecular Geometry / Shape and Bond Angles viral
Otras descripciones de Al Hco3 3 lo que puede ver
In this video we’ll write the correct name for Al(HCO3)3. To write the name for Al(HCO3)3 we’ll use the Periodic Table and follow some simple rules.
Because Al(HCO3)3 has a polyatomic ion we’ll also need to use a table of names for common polyatomic ions, in addition to the Periodic Table.
• Common Ion Table: breslyn.org/chemistry/naming/resources/common_ion_table.php
• Memorizing the Polyatomic ions: youtu.be/vepxhM_bZqk
—Keys for Naming Ternary Ionic Compounds—
1) Name the metal (the cation) as it appears on the Periodic Table.
Na+ = Sodium Mg2+ = Magnesium Al3+ = Aluminum
2) Find the polyatomic ion on the Common Ion Table and write the name.
Note: It is possible to have two polyatomic ions such as NH4NO3. In this case find and write both names as found on the Common Ion Table.
—————————–
For ionic compounds like Aluminum bicarbonate (or hydrogen carbonate), you should check to make sure the charges balance to make sure you have the right name.
Naming Resources:
• Simple Ionic Compounds: youtu.be/P2FZzCKb1K0
• Compounds with Polyatomic ions: youtu.be/eTNSij-GVHk
• Compounds with Transition Metals: youtu.be/T_vIspr_S20
• Molecular Compounds: youtu.be/ElhicLT-pCc
• Naming Acids: youtu.be/xqHWU5Vj19Q
For a complete tutorial on naming and formula writing for compounds, like Aluminum bicarbonate (or hydrogen carbonate) and more, visit:
breslyn.org/chemistry/naming
Drawing/writing done in InkScape ( InkScape.org). Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
Nuevo How to Balance Al(HCO3)3 = Al2O3 + CO2 + H2O – YouTube actualizado

Temas 两性物质(Al、AlO3、Al(OH)3、HCO3离子)分别与强酸强碱的反应方程式及离子方程式._百度知道 viral

Actualmente – Написать уравнения электролитической диссоциации Al(NO3)3, H2SO4, Zn(OH viral

Temas What Is The Conjugate Base Of Hco3 – slidesharetrick Nuevo

Ver CО2 sequestration in mining residues – probing heat effects associated viral

Aquí Напишите уравнения реакций электролитической диссоциации растворов más

Imprescindible Dalam darah terdapat campuran H2CO3 dan HCO3─ yang merupakan sistem viral

Imágenes Вычислите эквивалент и молярную массу эквивалента гидрокарбоната бария

Artículos What Is The Conjugate Base Of Hco3 – slidesharetrick

Fotos Напишите молекулярные и ионно-молекулярные уравнения реакций Ca(HCO3)2